��Ŀ����
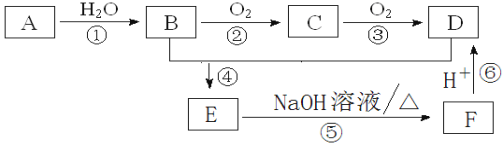
����Ŀ��ʵ���ҿ�������װ��![]() ��ȥ���ּг�����
��ȥ���ּг�����![]() ��ȡ
��ȡ![]() ����֤�����ʣ�
����֤�����ʣ�
(1)ʢװ![]() ����������Ϊ__________
����������Ϊ__________ ![]() ����ȱ�ٵIJ���������________����
����ȱ�ٵIJ���������________����![]() �Ļ�ѧ����ʽ_______��
�Ļ�ѧ����ʽ_______��
(2)װ��B������֮һ��ͨ���۲�������ݵĶ����ж�![]() ���ɵĿ��������е�Һ�����ѡ��____
���ɵĿ��������е�Һ�����ѡ��____![]() �����
�����![]() ��
��
a. ����![]() ��Һ b. ����
��Һ b. ����![]() ��Һ
��Һ
c. ����![]() ��Һ d. ����NaHSO3��Һ
��Һ d. ����NaHSO3��Һ
(3)��֤��������������Ե�װ����______������Ϊ_______________��
(4)Ϊ��֤��������Ļ�ԭ�ԣ���ַ�Ӧ��ȡ���Թ��е���Һ�ֳ����ݣ��ֱ��������ʵ�飺
����I�����һ����Һ�м���![]() ��Һ���а�ɫ�������ɣ�
��Һ���а�ɫ�������ɣ�
��������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ��
���������������Һ�м���![]() ��Һ��������ɫ������
��Һ��������ɫ������
���������������Ƿ���________![]() ����I������������������
����I������������������![]() �������Թ�D��������ˮ��Ϊ���Ը��������Һ��������Ӧ�����ӷ���ʽΪ____________________��
�������Թ�D��������ˮ��Ϊ���Ը��������Һ��������Ӧ�����ӷ���ʽΪ____________________��
(5)װ��F��������___________________![]()
���𰸡�Բ����ƿ �ƾ��� Cu+2H2SO4![]() CuSO4+SO2��+2H2O d C �е���ɫ�������� �� 5SO2+2MnO4- +2H2O=5SO42- + 2Mn2+ +4H+ ����δ��Ӧ�Ķ����������壬��ֹ��Ⱦ����
CuSO4+SO2��+2H2O d C �е���ɫ�������� �� 5SO2+2MnO4- +2H2O=5SO42- + 2Mn2+ +4H+ ����δ��Ӧ�Ķ����������壬��ֹ��Ⱦ����
��������
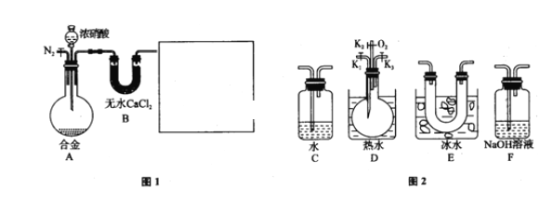
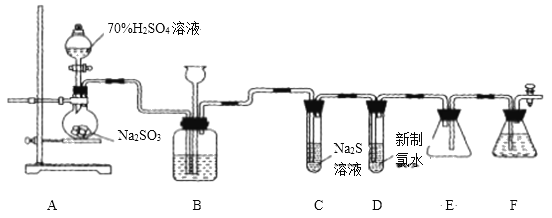
����ʵ��װ�ü�ʵ��ԭ������ʵ���е��������Ƽ�����Ҫ��ʵ�����ģ����ݶ�������������Լ���ԭ�Է���ʵ��ʵ�鷽���Ŀ����ԣ���д����ػ�ѧ����ʽ��
(1)��ͼ��ʾ��ʢװ�������Ƶ���������ΪԲ����ƿ�� Ũ������ͭ��Ӧ���ɶ���������Ҫ���ȣ����Ի�ȱ�ٵIJ��������Ǿƾ��ƣ���ѧ����ʽΪ��Cu+2H2SO4![]() CuSO4+SO2��+2H2O���ʴ�Ϊ��Բ����ƿ���ƾ��ƣ�Cu+2H2SO4
CuSO4+SO2��+2H2O���ʴ�Ϊ��Բ����ƿ���ƾ��ƣ�Cu+2H2SO4![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
(2)�����������ǿ��̼�ᣬ���Զ���������![]() ��Һ ��
��Һ ��![]() ��Һ��Ӧ���ɶ�����̼���壬����������
��Һ��Ӧ���ɶ�����̼���壬����������![]() ��Һ��Ӧ�������������ƣ��Ҷ�������������ˮ���������е�Һ�����ѡ��NaHSO3��Һ��
��Һ��Ӧ�������������ƣ��Ҷ�������������ˮ���������е�Һ�����ѡ��NaHSO3��Һ��
(3)����������һ���������ԣ�������Na2S��Ӧ���ɵ�����������֤��������������Ե�װ����C������Ϊ�е���ɫ�������ɣ�
(4)����������л�ԭ�ԣ���������ˮ����������ԭ��Ӧ���������
����I�����һ����Һ�м���![]() ��Һ���а�ɫ�������ɣ���ˮ�б����ʹ��������ӣ����������Ȼ���������������֤��������Ļ�ԭ�ԣ���I��������
��Һ���а�ɫ�������ɣ���ˮ�б����ʹ��������ӣ����������Ȼ���������������֤��������Ļ�ԭ�ԣ���I��������
��������ڶ�����Һ�м���Ʒ����Һ����ɫ��ȥ��������ˮ��Ư���ԣ�����ʹƷ����ɫ������IIҲ������֤��������Ļ�ԭ�ԣ���II��������
���������������Һ�м���![]() ��Һ��������ɫ��������ʱ��ҺΪ���ԣ������İ�ɫ����Ӧ��Ϊ���ᱵ��˵������������ˮ��������֤�˶�������Ļ�ԭ�ԣ���III������
��Һ��������ɫ��������ʱ��ҺΪ���ԣ������İ�ɫ����Ӧ��Ϊ���ᱵ��˵������������ˮ��������֤�˶�������Ļ�ԭ�ԣ���III������
���Ը��������ҺҲ����ǿ�����ԣ������������������������ᣬ��������ԭ���������ӣ�������Ӧ�����ӷ���ʽΪ��5SO2+2MnO4- +2H2O=5SO42- + 2Mn2+ +4H+��
(5)���������ж���װ��F�������ǣ�����δ��Ӧ�Ķ����������壬��ֹ��Ⱦ������

����Ŀ���״�����Ҫ�Ļ���ԭ�ϡ����úϳ���(��Ҫ�ɷ�ΪCO��CO2��H2)�ڴ����������ºϳɼ״������ܷ����ķ�Ӧ���£�
��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����H1
CH3OH(g)+H2O(g)����H1
��CO2(g)+H2(g)![]() CO(g)+H2O(g)����H2
CO(g)+H2O(g)����H2
��CH3OH(g)![]() CO(g)+2H2(g)����H3
CO(g)+2H2(g)����H3
�ش��������⣺
(1)��֪��Ӧ������ػ�ѧ�������������£�
��ѧ�� | H��H | C��O | C��O | H��O |
E/kJ��mol��1 | 436 | 803 | 1076 | 465 |
�ɴ˼�����H2��____kJ��mol��1����֪��H3����99kJ��mol��1������H1��____kJ��mol��1��
(2)һ�������ĺϳ�����װ�д����ķ�Ӧ���з�Ӧ12Сʱ����ϵ�м״��IJ��ʺʹ����Ĵ��������¶ȵĹ�ϵ��ͼ��ʾ��

���¶�Ϊ470Kʱ��ͼ��P��____(������������������)����ƽ��״̬����490K֮ǰ���״��������¶����߶�����490K֮�״��������¶����߶���С��ԭ��ֱ���____��
��һ������״����ʵĴ�ʩ��____��
A������ѹǿ B�������¶� C��ѡ����ʴ��� D�������������
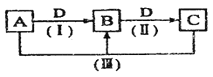
(3)��ͼΪһ��������CO2/H2��CO/H2��CO/CO2/H2�����¼״������������¶ȵĹ�ϵ��

��490Kʱ����������a��c�жϺϳɼ״��ķ�Ӧ������____(����I������II��)��
����CO2![]() CO
CO![]() CH3OH II��CO
CH3OH II��CO![]() CO2
CO2![]() CH3OH��H2O
CH3OH��H2O
��490Kʱ������a������b��ȣ�CO�Ĵ���ʹ�״�����������������ѧ�붯��ѧ�Ƕȣ�����Ϸ�Ӧ�����ڷ���ԭ��____��