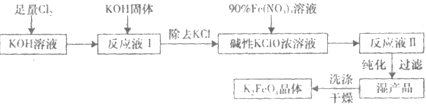
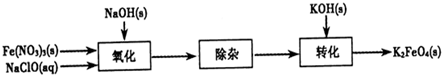
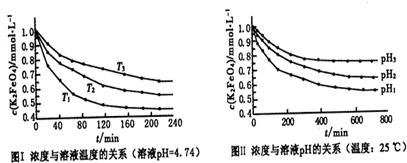
��Ŀ����
������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ����������������������

��1����Ӧ��Ӧ���¶Ƚϵ͵�����½��С������¶Ƚϸ�ʱKOH��Cl2��Ӧ���ɵ���KClO3��д�����¶Ƚϸ�ʱKOH ��Cl2��Ӧ�Ļ�ѧ����ʽ______________________������Ӧ��ת��5mol����ʱ�����ĵ�������
___________mol��
��2���ڷ�ӦҺI�м���KOH�����Ŀ����___________�����ţ���
A���뷴ӦҺI�й�����Cl2������Ӧ�����ɸ����KClO
B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ����
C��Ϊ��һ����Ӧ�ṩ���ԵĻ���
D��ʹKClO3ת��ΪKClO
��3������ҺII�з����K2FeO4�����и���ƷKNO3��KCl����Ӧ���з��������ӷ�Ӧ����ʽΪ
___________________________��
��4������ж�K2FeO4�����Ѿ�ϴ�Ӹɾ�______________________��
��5���������(K2FeO4)��Ϊˮ��������һ���ŵ�������ˮ��Ӧ���ɽ����������ʣ������ӷ�Ӧ�ǣ�
____FeO42-+____H2O��_____Fe(OH)3(����)+_____O2��+______��ɲ���ƽ����ʽ�����������ӷ���ʽд���·���______________________��
___________mol��
��2���ڷ�ӦҺI�м���KOH�����Ŀ����___________�����ţ���
A���뷴ӦҺI�й�����Cl2������Ӧ�����ɸ����KClO
B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ����
C��Ϊ��һ����Ӧ�ṩ���ԵĻ���
D��ʹKClO3ת��ΪKClO
��3������ҺII�з����K2FeO4�����и���ƷKNO3��KCl����Ӧ���з��������ӷ�Ӧ����ʽΪ
___________________________��
��4������ж�K2FeO4�����Ѿ�ϴ�Ӹɾ�______________________��
��5���������(K2FeO4)��Ϊˮ��������һ���ŵ�������ˮ��Ӧ���ɽ����������ʣ������ӷ�Ӧ�ǣ�
____FeO42-+____H2O��_____Fe(OH)3(����)+_____O2��+______��ɲ���ƽ����ʽ�����������ӷ���ʽд���·���______________________��
��1��6KOH+3Cl2==KClO3+5KCl+3H2O��3
��2��AC
��3��2Fe3++3ClO-+10OH-==2FeO42-+3Cl-+5H2O
��4��ȡ���һ�ε�ϴ��Һ������������Һ���ް�ɫ�������ѱ�ϴ��
��5��4FeO42-+10H2O=4Fe(OH)3(����)+3O2��+8OH-
��2��AC
��3��2Fe3++3ClO-+10OH-==2FeO42-+3Cl-+5H2O
��4��ȡ���һ�ε�ϴ��Һ������������Һ���ް�ɫ�������ѱ�ϴ��
��5��4FeO42-+10H2O=4Fe(OH)3(����)+3O2��+8OH-

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ