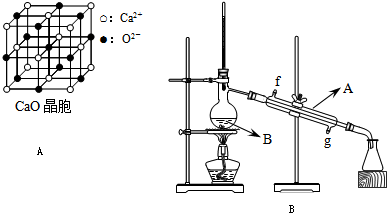
��Ŀ����
��a��b����������Ҽ����ȵ��ܱ�������a�����ݻ����䣬b�еĻ����������ƶ����Ա�������ѹǿ��ȣ�����ͬ�����½�3 mol��A��1 mol��B�ֱ�ͬʱ�����a��b�������У�������Ӧ��3A(g)��B(g)![]() 2C(g)��D(g)
2C(g)��D(g)
(1)�ﵽƽ��ʱ��a��A��Ũ��ΪM mol/L��A��ת����ΪN��b��A��Ũ��Ϊm mol/L��A��ת����Ϊn����M________m��N________n(��������������Ƚ�)
(2)�����¶Ȳ��䣬��������ȷֱ����a��b����������ƽ���a��A��Ũ��ΪM mol��L��1����________��
A��6 mol��A��2 mol��B
B��3 mol��A��2 mol��C
C��2 mol��C��1 mol��B��1 mol��D
D��2 mol��C��1 mol��D
E��1.5 mol��C��0.5 mol��B��1 mol��C��0.5 mol��D
�𰸣���,��;D,E

��ϰ��ϵ�д�
�����Ŀ

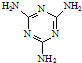 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 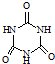 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ�� 2C(g)��D(g)
2C(g)��D(g) 2C(g)��D(g)
2C(g)��D(g) 2C(g)��D(g)
2C(g)��D(g)