��Ŀ����
����A��I���ɶ�����Ԫ����ɵij������ʣ�����������D��������F�Dz��������������E�����뵼����ϡ�����������⣺ 
��1��������A�ĵ���ʽ��________________��������F�Ļ�ѧʽ��________________��
��2����Ӧ1�ڹ�ҵ�ϵ���;��___________��д��������F�뵥��B��Ӧ�IJ����������G��Ӧ�Ļ�ѧ����ʽ___________________________________��
��3������E��Ԫ�����ڱ��е�λ����___________������H�͵���E�����γɺ�������I��ͬ�ľ��壬�����۵�ߵ���___________��ԭ����________________________________��
��4������B�͵���C��һ�������¿����ԭ��ء���KOH��aq)������ʡ������ԭ��صĸ�����Ӧ����ʽ��___________________________________��
��1��![]() NO
NO
��2����������������ĵ�һ����Ӧ 3NO2+H2O====2HNO3+NO
��3����3���ڵڢ�A�� ���ʯ r(C��C��С��r(Si��Si)���Ͽ�C��C����Ҫ�������ȶϿ�Si��Si����Ҫ��������
��4��2H2+4OH--4e-====4H2O
������F�Dz����������������NO��CO��E�����뵼����ϣ�ֻ����Si�����ɻ�����A+����B����![]() ������F+������G������֪��F��ΪNO�����ݰ��Ĵ�������Ӧ��֪��AΪNH3��BΪO2��GΪH2O��
������F+������G������֪��F��ΪNO�����ݰ��Ĵ�������Ӧ��֪��AΪNH3��BΪO2��GΪH2O��

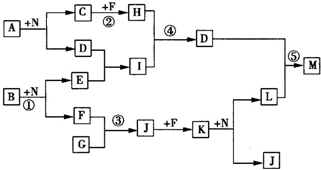 ���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��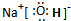
 ���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
