��Ŀ����
|
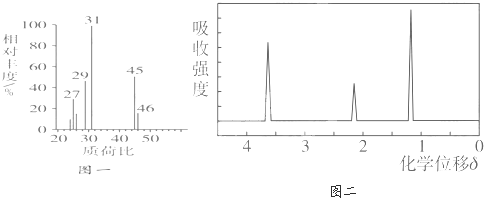
�л���A����Է�������Ϊ102�����к�������������Ϊ31.4������ȫȼ��ֻ���ɶ�����̼��ˮ����n(CO2)��n(H2O)���л���֮����ת������(���ַ�Ӧ������)������˵����ȷ����
| |
A�� |
�л���C��B��Ħ��������� |
B�� |
��M(X)��ʾ�л����Ħ���������� 2M(D)��18��M(B)��M(E) |
C�� |
�л���A�ܷ���������Ӧ���칹�������� |
D�� |
�л���E�ܷ��� ȡ������ȥ���к͵ȷ�Ӧ |
�𰸣�AB

��ϰ��ϵ�д�
�����Ŀ

 ����ij��̼�����������ϣ�
����ij��̼�����������ϣ� ����ԭ��֮һ��
����ԭ��֮һ��






 ��
�� ��
�� ��
��

 ����ij�ϳ�������
����ij�ϳ�������  ����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
����ԭ��֮һ����صĺϳ�·������ͼ��ʾ��ijЩ����������ȥ����
 ��R1��R2��R3����������
��R1��R2��R3����������



 ��X�Ľṹ��ʽΪ
��X�Ľṹ��ʽΪ