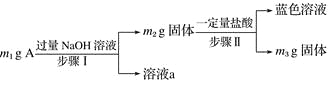
��Ŀ����
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ�����A��B��A��D�����ڱ���λ�����ڣ�Aԭ�Ӻ���������δ�ɶԵ��ӣ�BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض���Cԭ����ͬ����ԭ���а뾶���(ϡ���������)��E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ������������������Ϣ���ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C��D����Ԫ�ص縺���ɴ�С����˳��Ϊ________________________________________��
��2��B���⻯��Ľṹ��____________________________����ռ乹��Ϊ____________________________________________________��
��3��E��������Ų�ʽ��__________________��E��ij�ֻ�����Ľṹ����ͼ��ʾ��

���������ð�����ѧ���ͷ��Ӽ�����������˻������и������Ӽ�����������______________________��
��4��A��B����̬�⻯��ķе�________���ߣ�A��D����̬�⻯��ķе�________���ߡ�
��5��A���ȶ��������У�����ԭ�ӵ��ӻ�����Ϊ________���ռ乹��Ϊ________��
���𰸡� N>C>Si>Na ![]() ������ 1s22s22p63s23p63d104s1��[Ar]3d104s1 ���Ӽ������ۼ�����λ������� NH3 SiH4 sp�ӻ� ֱ����
������ 1s22s22p63s23p63d104s1��[Ar]3d104s1 ���Ӽ������ۼ�����λ������� NH3 SiH4 sp�ӻ� ֱ����
��������������Ҫ������ӽṹ��A��B��D�����λ��Ϊ![]() ������ΪB�ĵ�һ�����ܱ�ͬ�������ڵ�����Ԫ�ش�����BΪ������ṹ��BΪN��AΪC��DΪSi��CΪNa��EΪ�������ڵ�Ԫ�أ������Ϊ1�����ӣ���������Ӳ����������E�ĵ����Ų�ʽΪls22s22p63s23p63d104s1��EΪCu��
������ΪB�ĵ�һ�����ܱ�ͬ�������ڵ�����Ԫ�ش�����BΪ������ṹ��BΪN��AΪC��DΪSi��CΪNa��EΪ�������ڵ�Ԫ�أ������Ϊ1�����ӣ���������Ӳ����������E�ĵ����Ų�ʽΪls22s22p63s23p63d104s1��EΪCu��
(1)A��B��C��D�ֱ�ΪC��N��Na��Si�����ݵ縺�Եĵݱ���ɿ�֪���縺��N>C>Si>Na��(2)B����̬�⻯��ΪNH3������ԭ��N��sp3�ӻ����ռ乹��Ϊ�����Ρ�
(3)EΪCu��������Ų�ʽΪ1s22s22p63s23p63d104s1������ͼʾ���ж�H2O���Ӻ�Cu2���������λ����ͬʱˮ����֮�仹���������H2O�����ڴ��ڹ��ۼ����û����ﻹ���������ӣ��������Ӽ���
(4)A��B����̬�⻯��ֱ�ΪCH4��NH3����е�ߵ�ΪNH3>CH4(��NH3����֮��������)��A��D����̬�⻯��ֱ�ΪCH4��SiH4����������ɺͽṹ���ƣ�SiH4����Է�����������CH4���ʷе�SiH4>CH4��
(5)CO2��Cԭ��sp�ӻ���CO2���ӳ�ֱ���Ρ�

����Ŀ����һ�����ܱ�������,������Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
T�� | 700 | 800 | 850 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�Իش���������:
��1��������ӦΪ____(��������������������)��Ӧ,�����¶�,ƽ����______ (��������Ӧ�������淴Ӧ��) �����ƶ���
��2��ij�¶���,�����Ϊ2L�ĺ����ܱ�������ͨ��2molCO2(g)��4molH2(B)��������Ӧ,5minʱ��Ӧ�ﵽƽ��,���CO2(g)��ת������75%��
��v(H2O)=______mol��L-1��min-l��
�ڸ��¶��·�Ӧ��ƽ�ⳣ��K=______.
��3������ˮú���Ĺ�������:
��C(s)+CO2(g) ![]() 2CO(g)��H1
2CO(g)��H1
��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H2
CO2(g)��H2(g) ��H2
�۷�Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��
CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��