��Ŀ����
17����1�������г�����һЩԭ�ӵ�2p�ܼ���3d�ܼ��е����Ų�����������жϣ���ЩΥ��������ԭ���ۣ���ЩΥ���˺��ع���ܣ�
��2��ijԪ�صļ���̬�����ȶ�״̬��ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s13p33d2�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p4��������������Ӧˮ����Ļ�ѧʽ��H2SO4��
��3�������ж����ԭ�ӵ�ԭ�ӹ������������ɵ͵���˳�����Т٣��ܣ��ޣ��ۣ��ڣ��ݣ�
��2s����3d����4s����3s����4p����3p��
���� ��1������������ԭ����ÿ��ԭ�ӹ�������ֻ������2������״̬�෴�ĵ��ӣ����ع�����ͬһ�������Dz����Ų��ĵ��ӣ����Ǿ���ռ�ݲ�ͬ�Ĺ����������������ͬ��
��2��ͬһԪ�ص�ԭ���У���̬�ͼ���̬ԭ�ӵĺ������������ȣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��������Ԫ���У�Ԫ�ص�����ϼ۵��������������Ӷ�ȷ��������������Ӧ��ˮ���
��3����ͬ�㲻ͬ�ܼ���ԭ�ӹ�������ĸߵ�˳��1s��2s��2p��3s��3p��4s��3d��4p��5s��4d��5p��6s��4f����
��� �⣺��1��ͬһ��ԭ�ӹ���в�Ӧ������״̬��ͬ�ĵ��ӣ���Υ��������ԭ����
���ڻ�̬ԭ�ӣ�������������ͬ�Ĺ�����Ų�ʱ���������ܷ�ռ��ͬ�Ĺ����������״̬��ͬ����Υ���˺��ع���
�ʴ�Ϊ���ۣ��ܣ�
��2��3p����С��3d������̬Ϊ1s22s22p63s13p33d2����̬ӦΪ1s22s22p63s23p4��ԭ�Ӻ��������Ϊ16����������Ϊ16����Ԫ��Ϊ��Ԫ�أ�������������Ӧˮ����Ļ�ѧʽ��H2SO4��
�ʴ�Ϊ��1s22s22p63s23p4��H2SO4��
��3���ܲ�Խ�ߣ�����Խ��ͬһ�ܲ㣬p�ܼ���������s�ܼ����������ܼ�������֪������Ϊ4s��3d��4p����ͬ�㲻ͬ�ܼ���ԭ�ӹ�������ĸߵ�˳��2s��3s��3p��4s��3d��4p���ʴ�Ϊ���٣��ܣ��ޣ��ۣ��ڣ��ݣ�
���� ���⿼���������Ų����ɣ�ԭ�ӹ���ܼ�˳����Ŀ�Ѷ��еȣ�ע����ո�������ԭ�������ع�������ݣ�

| A | B | ||||||
| D | E | F | |||||
| C | I | G | H |

��2���ȶ��ԱȽϣ�D���⻯�G���⻯�����ڡ������ڡ���С�ڣ�
��3����һ�������£�A��E���γ�һ�ּ�������ˮ����̬����������ʽΪ

��4�������ϱ���������ĸ������ijһ��Ԫ�أ��䵥��ͨ�뵽��ɫʯ����Һ�е�����Ϊ�ȱ�����ɫ��д���йص����ӷ���ʽCl+H2O=H++Cl-+HClO
��5�������ۡ��ߺ����˷ɴ�����Ҫ��һ�ֻ����������պ���Ա������CO2��������Ӧ�������ϱ��е�Ԫ����ɵģ��û�ѧ����ʽ��ʾ����ԭ����2Na2O2+2CO2�T2Na2CO3+O2�ɴ�����Ҫ����һ���ʺϺ���Ա������˹�̬��������Ӧ���������г���һ��ϡ�����壬������ĽṹʽΪN��N��
| A�� | 32 | B�� | 20 | C�� | 16 | D�� | 18 |
 ��ʾ̼ԭ�ӣ�
��ʾ̼ԭ�ӣ� ��ʾ̼̼������δ�����ļ���ʾ̼ԭ������ԭ��������������˵����ȷ���ǣ�������
��ʾ̼̼������δ�����ļ���ʾ̼ԭ������ԭ��������������˵����ȷ���ǣ�������
| A�� | a��c��Ϊͬ���칹�� | B�� | b��ʹ���Ը��������Һ��ɫ | ||
| C�� | b��c��Ϊͬϵ�� | D�� | ������dΪ���� |
| A�� | c��X2��=0.15 mol•L-1 | B�� | c��Y2��=0.7 mol•L-1 | ||
| C�� | c��Y2��=0.3 mol•L-1 | D�� | c��Q2��=0.6 mol•L-1 |
| A�� |  Ũ�����ϡ�� | B�� |  ʵ������ȡCO2 | C�� |  ���Ļ��� | D�� |  ��ֹ���� |
 X��Y��Z��RΪ������Ԫ�أ�ԭ��������������X��һ�ֵ�������Ȼ������Ӳ�����ʣ�Y�ĵ����ڿ����к�����ߣ�Z ���������dz��������������R ��̬ԭ�������ɶԵ��ӵ���Ŀ��δ�ɶԵ��� ����Ŀ��ȣ�
X��Y��Z��RΪ������Ԫ�أ�ԭ��������������X��һ�ֵ�������Ȼ������Ӳ�����ʣ�Y�ĵ����ڿ����к�����ߣ�Z ���������dz��������������R ��̬ԭ�������ɶԵ��ӵ���Ŀ��δ�ɶԵ��� ����Ŀ��ȣ� 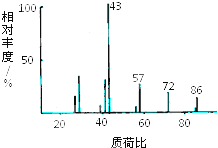 ij�л��ﺬ��C��H��O����Ԫ�أ���������ͼ��ʾ����4.3g���л�����O2�г��ȼ�գ�ʹ����������ͨ������Ũ����ͼ�ʯ�ң�Ũ��������2.7g����ʯ������8.8g����
ij�л��ﺬ��C��H��O����Ԫ�أ���������ͼ��ʾ����4.3g���л�����O2�г��ȼ�գ�ʹ����������ͨ������Ũ����ͼ�ʯ�ң�Ũ��������2.7g����ʯ������8.8g����