��Ŀ����
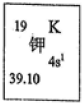 �ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺
�ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺��1����ͼ��ʾΪԪ�����ڱ��м�Ԫ�ؿ�ͼ�����ݡ�39.10����ʾ����
��2������Ԫ���У������ӵ��Ӳ�ṹ���ԭ����ͬ�������Ӱ뾶����Ԫ�أ���ԭ�Ӻ�����
��3��������Ӧ�������У����ڷǵ���ʵ���
��4��������ѧ����ʽ��Ԫ���У�����ͬ����Ԫ�صķǽ�������ǿ������˳��Ϊ
A������������Ӧˮ��������� B����̬�⻯��ķе�
C��������������Ӧ�����׳̶� D������������γɵĻ�������Ԫ�صĻ��ϼ�
��5����������Ӧ�У���1.5mol��ԭ��
��������1��KԪ�ص����ԭ������Ϊ39.10������Ԫ��������=���Ӳ�������������������������
��2������Ԫ����S2-���Ӱ뾶������������ԭ����д���������Ų�ʽ������ȷ���ܼ���Ŀ��������������������Ӧ��������HCl�����
��3��������̼���ڷǵ���ʣ�������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ��е������K2S���������ӻ�����ɼ������������ӹ��ɣ�
��4��ͬ������ԭ����������Ԫ�صķǽ�������ǿ��
A����Ԫ��û�к����
B���������ʲ��ܱȽϷǽ�����ǿ����
C�����ʵĽṹ��ͬ������˵��Ԫ�صķǽ����ԣ�
D���������б��ָ��۵�Ԫ�أ��Լ��ϵ��ӵ���������ǿ���ǽ����Ը�ǿ��
��5����ԭ��ʧȥ���ӣ�����Ԫ�ػ��ϼ����ߣ���ԭ��ΪC����ϻ��ϼ۱仯����ת�Ƶ��ӣ���ԭ����ΪK2S��N2�����ݷ���ʽ��������K2S��N2�����ʵ������ٸ���m=nM���㣮
��2������Ԫ����S2-���Ӱ뾶������������ԭ����д���������Ų�ʽ������ȷ���ܼ���Ŀ��������������������Ӧ��������HCl�����
��3��������̼���ڷǵ���ʣ�������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ��е������K2S���������ӻ�����ɼ������������ӹ��ɣ�
��4��ͬ������ԭ����������Ԫ�صķǽ�������ǿ��
A����Ԫ��û�к����
B���������ʲ��ܱȽϷǽ�����ǿ����
C�����ʵĽṹ��ͬ������˵��Ԫ�صķǽ����ԣ�
D���������б��ָ��۵�Ԫ�أ��Լ��ϵ��ӵ���������ǿ���ǽ����Ը�ǿ��
��5����ԭ��ʧȥ���ӣ�����Ԫ�ػ��ϼ����ߣ���ԭ��ΪC����ϻ��ϼ۱仯����ת�Ƶ��ӣ���ԭ����ΪK2S��N2�����ݷ���ʽ��������K2S��N2�����ʵ������ٸ���m=nM���㣮
����⣺��1��39.10��KԪ�ص����ԭ��������KԪ��ԭ����Χ�����Ų�Ϊ4s1�����Ӳ���Ϊ4������������Ϊ1�����ڵ������ڢ�A�壬
�ʴ�Ϊ��KԪ�ص����ԭ���������������ڢ�A�壻
��2������Ԫ����S2-���Ӱ뾶������������Ų�ʽΪ1s22s22p63s23p6����5�ֲ�ͬ�ܼ��ĵ��ӣ�������������������Ӧ��������HCl�����ᣬ��Ӧ����ʽΪ��H2SO3+Cl2+H2O=H2SO4+2HCl��
�ʴ�Ϊ��5��H2SO3+Cl2+H2O=H2SO4+2HCl��
��3��������̼���ڷǵ���ʣ�������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O���е������K2S���������ӻ�����ɼ������������ӹ��ɣ������ʽΪ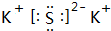 ���ʴ�Ϊ��O=C=O��
���ʴ�Ϊ��O=C=O��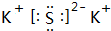 ��
��
��4��C��N��Oͬ���ڣ�ͬ������ԭ����������Ԫ�صķǽ�������ǿ���ʷǽ�����O��N��C��
A����Ԫ��û�к����ᣬ����������۱Ƚ���Ԫ����C��NԪ�صķǽ����ԣ���A����
B���е������������ʣ����ܱȽϷǽ�����ǿ������B����
C��������H2��Ӧ�����׳̶ȣ�˵�����ʵ�������ǿ�������ʵĽṹ��ͬ������˵��Ԫ�صķǽ����ԣ���C����
D��Ԫ�صĻ��ϼۣ�˵�����õ��Ӷ�ƫ�Ʒ��������б��ָ��۵�Ԫ�أ��Լ��ϵ��ӵ���������ǿ���ǽ����Ը�ǿ����D��ȷ��
�ʴ�Ϊ��O��N��C��D��
��5����ԭ��ʧȥ���ӣ�����Ԫ�ػ��ϼ����ߣ���ԭ��ΪC��CԪ�ػ��ϼ���0������Ϊ+4����1.5mol��ԭ����Ӧ��ʧȥ����Ϊ1.5mol����4-0��=6mol����ԭ����ΪK2S��N2���ɷ���ʽ��֪����0.5molK2S��0.5molN2���ʻ�ԭ���������=0.5mol��110g/mol+0.5mol��28g/mol=69g��
�ʴ�Ϊ��ʧȥ��6��69��
�ʴ�Ϊ��KԪ�ص����ԭ���������������ڢ�A�壻
��2������Ԫ����S2-���Ӱ뾶������������Ų�ʽΪ1s22s22p63s23p6����5�ֲ�ͬ�ܼ��ĵ��ӣ�������������������Ӧ��������HCl�����ᣬ��Ӧ����ʽΪ��H2SO3+Cl2+H2O=H2SO4+2HCl��
�ʴ�Ϊ��5��H2SO3+Cl2+H2O=H2SO4+2HCl��
��3��������̼���ڷǵ���ʣ�������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O���е������K2S���������ӻ�����ɼ������������ӹ��ɣ������ʽΪ
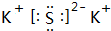 ���ʴ�Ϊ��O=C=O��
���ʴ�Ϊ��O=C=O��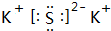 ��
����4��C��N��Oͬ���ڣ�ͬ������ԭ����������Ԫ�صķǽ�������ǿ���ʷǽ�����O��N��C��
A����Ԫ��û�к����ᣬ����������۱Ƚ���Ԫ����C��NԪ�صķǽ����ԣ���A����
B���е������������ʣ����ܱȽϷǽ�����ǿ������B����
C��������H2��Ӧ�����׳̶ȣ�˵�����ʵ�������ǿ�������ʵĽṹ��ͬ������˵��Ԫ�صķǽ����ԣ���C����
D��Ԫ�صĻ��ϼۣ�˵�����õ��Ӷ�ƫ�Ʒ��������б��ָ��۵�Ԫ�أ��Լ��ϵ��ӵ���������ǿ���ǽ����Ը�ǿ����D��ȷ��
�ʴ�Ϊ��O��N��C��D��
��5����ԭ��ʧȥ���ӣ�����Ԫ�ػ��ϼ����ߣ���ԭ��ΪC��CԪ�ػ��ϼ���0������Ϊ+4����1.5mol��ԭ����Ӧ��ʧȥ����Ϊ1.5mol����4-0��=6mol����ԭ����ΪK2S��N2���ɷ���ʽ��֪����0.5molK2S��0.5molN2���ʻ�ԭ���������=0.5mol��110g/mol+0.5mol��28g/mol=69g��
�ʴ�Ϊ��ʧȥ��6��69��
���������⿼��Ԫ�����ڱ���Ԫ�������ɡ����û�ѧ���������ԭ��Ӧ�ȣ��ѶȲ���4����֤���ǽ�����ǿ����ʵΪ�״��㣬ѧ����������Ԫ��û�к����ἰ���ʵĽṹ��

��ϰ��ϵ�д�
�����Ŀ
 �����б���ԭ��Ԫ����_________����������Ԫ����_________����������_________����ԭ����_________������������_________����ԭ������_________��
�����б���ԭ��Ԫ����_________����������Ԫ����_________����������_________����ԭ����_________������������_________����ԭ������_________��