��Ŀ����
2������˵����ȷ���ǣ�������| A�� | ���ʵ���Ũ����ͬ��NaCl��Һ��NaClO��Һ��NaCl��Һ�����ӵ���Ũ�ȴ���NaClO��Һ�����ӵ���Ũ�� | |
| B�� | 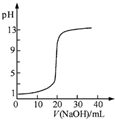 ��ͼ��ʾ0.1000mol•L-1NaOH��Һ�ζ�20.00mL0.1000mol•L-1CH3COOH��Һ���õ��ĵζ����� | |
| C�� | �к�ͬ�����ͬŨ�ȵ�����ʱ������pH��ͬ�İ�ˮ���������ƺ�����������Һ�������С��ϵ�ǣ���ˮ�������������������� | |
| D�� | ��NaH2PO4ˮ��Һ�д��ڹ�ϵ��C��H3PO4��+C��H+���TC��HPO42-��+C��PO43-��+C��OH-�� |
���� A�������Ȼ�����Һ�еĵ���غ��֪��c��Na+��+c��H+��=c��Cl-��+c��OH-����NaClO��Һ������c��Na+��+c��H+��=c��ClO-��+c��OH-����NaCl��Һ�����ԣ�NaClO��Һ�ʼ��ԣ���NaClO��Һ��c��H+����С������NaCl��Һ�����ӵ���Ũ�ȴ���NaClO��Һ�����ӵ���Ũ�ȣ��ݴ˽����жϣ�
B.0.1000mol•L-1CH3COOH��Һ�д��ڵ���ƽ�⣻
C��һˮ�ϰ����������Һ�в��ֵ��룬��ˮ��Ũ�ȴ������������������ӵ�Ũ�ȣ�pHֵ��ȡ������ͬ��ǿ��������������ʵ�����ͬ��
D�������Һ�е���غ�������غ���������ϵʽ��
��� �⣺A���ɵ���غ��֪��NaCl��Һ��c��Na+��+c��H+��=c��Cl-��+c��OH-����NaClO��Һ��c��Na+��+c��H+��=c��ClO-��+c��OH-����NaCl��Һ�����ԣ�NaClO��Һ�ʼ��ԣ���NaClO��Һ��c��H+����С����NaCl��Һ�����ӵ���Ũ�ȴ���NaClO��Һ�����ӵ���Ũ�ȣ���A��ȷ��
B.20.00mL0.1000mol•L-1CH3COOH��Һ�д��ڵ���ƽ�⣬������Ũ��С��0.1mol/L����ҺPH��1����B����
C��һˮ�ϰ����������Һ�в��ֵ��룬��ˮ��Ũ�ȴ������������������ӵ�Ũ�ȣ�pH��ͬ��������Һ�����������ӵ�Ũ����ͬ����pHֵ��ȡ������ͬ��ǿ�����ĵ���������ʵ�����ͬ�����ڰ�ˮ��Ũ�ȴ�����ˮ���ĵ�����������C����
D����Һ�е���غ�Ϊ��C��H+��+C��Na+��=C��OH-��+C��H2PO4-��+2C��HPO42-��+3C��PO43-������Һ�д��������غ�C��Na+��=C��H2PO4-��+C��HPO42-��+C��PO43-��+C��H3PO4�����������õ�C��H3PO4��+C��H+���TC��HPO42-��+2C��PO43-��+C��OH-������D����
��ѡA��
���� ���⿼������Ӧ���������Һ�е���غ㡢�����غ㡢������ʵ���ƽ���֪ʶ�㣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ThԪ�ص���������231 | B�� | ${\;}_{92}^{233}$U��������Ϊ92 | ||
| C�� | 232Th��Ħ������Ϊ232 | D�� | 230Th��232Th�Ļ�ѧ������ͬ |
| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
| A�� | ������ҩ����ϩ�ƷӵĽṹ��ʽΪ��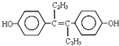 �����ķ���ʽ�ǣ�C18H22O2 �����ķ���ʽ�ǣ�C18H22O2 | |
| B�� | �л���CH3CO18OH��C2H5OH��Ũ����������²���ΪCH3CO18OC2H5��H2O | |
| C�� | ʵ��֤ʵ ��ʹ������Ȼ�̼��Һ��ɫ��˵���÷����д���̼̼˫�� ��ʹ������Ȼ�̼��Һ��ɫ��˵���÷����д���̼̼˫�� | |
| D�� | 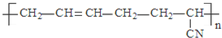 �ĵ�����CH3-C��C-CH3��CH2=CH-CN �ĵ�����CH3-C��C-CH3��CH2=CH-CN |
| A�� | ������ɫ���� | B�� | ���������л��ܼ� | ||
| C�� | H2������������ | D�� | HAt��HCl�ȶ� |
��ʯ����Ҵ����������Ƣ���������þ��̼��ƣ�
| A�� | �٢ۢܢݢ� | B�� | �ڢۢܢ� | C�� | �٢ڢܢݢ� | D�� | ȫ�� |
| A�� | ����±������������ˮ����ˮ�ص�Һ�� | |
| B�� | ����±�������ʵ������¶��ܷ�����ȥ��Ӧ | |
| C�� | ����±����������±��ԭ�� | |
| D�� | ����±��������ͨ��ȡ����Ӧ�Ƶõ� |
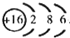
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�