��Ŀ����
��������Ҫ�Ļ������ϣ����������������������ǹ�ҵ���������Ҫ��Ӧ֮һ��
��1����0.050 mol SO2(g)��0.030 mol O2(g)�����ݻ�Ϊ1 L���ܱ������У���Ӧ2SO2(g)+O2(g) 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L��
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L��
�� ��ƽ��Ƕȷ������ù���O2��Ŀ����____________��
�� ����������·�Ӧ��ƽ�ⳣ��K��___________��
�� ��֪��K(300��)��K(350��)���÷�Ӧ��________�ȷ�Ӧ������Ӧ�¶����ߣ�SO2��ת����_______�����������С�����䡱����
��1����0.050 mol SO2(g)��0.030 mol O2(g)�����ݻ�Ϊ1 L���ܱ������У���Ӧ2SO2(g)+O2(g)
 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L��
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)��0.040 mol/L�� �� ��ƽ��Ƕȷ������ù���O2��Ŀ����____________��
�� ����������·�Ӧ��ƽ�ⳣ��K��___________��
�� ��֪��K(300��)��K(350��)���÷�Ӧ��________�ȷ�Ӧ������Ӧ�¶����ߣ�SO2��ת����_______�����������С�����䡱����

��2��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ(P)�Ĺ�ϵ��ͼ1��ʾ��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A)_______K(B)�����������������������ͬ����
��3����ͼ2��ʾ�������¶Ȳ��䣬��2 mol SO2��1 mol O2����������У���4 mol SO3�����������У�����K�����ƶ�����ʱ���ƻ���P��ʹ�ҵ��ݻ�Ϊ��2����
�����ƶ�����P��ʹ�ҵ��ݻ��ͼ���ȣ��ﵽ��ƽ��ʱ��SO3�����������______�ҡ�
������������ѹǿ���䣬��ס���������ͨ��������ĺ������ﵽ��ƽ��ʱ��SO3�����������____�ҡ�
��3����ͼ2��ʾ�������¶Ȳ��䣬��2 mol SO2��1 mol O2����������У���4 mol SO3�����������У�����K�����ƶ�����ʱ���ƻ���P��ʹ�ҵ��ݻ�Ϊ��2����
�����ƶ�����P��ʹ�ҵ��ݻ��ͼ���ȣ��ﵽ��ƽ��ʱ��SO3�����������______�ҡ�
������������ѹǿ���䣬��ס���������ͨ��������ĺ������ﵽ��ƽ��ʱ��SO3�����������____�ҡ�
��1������߶��������ת���ʣ���1600���۷��ȣ���С
��2��=
��3���٣����ڣ�
��2��=
��3���٣����ڣ�

��ϰ��ϵ�д�
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ




 +2H2O
+2H2O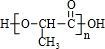 +��n-1��H2O
+��n-1��H2O




 �������룺���ڡ����ڡ�С�ڣ�
�������룺���ڡ����ڡ�С�ڣ�