��Ŀ����
��8�֣�CO��CH4��Ϊ�����Ŀ�ȼ������
��1���������CO��CH4����ͬ�����·ֱ���ȫȼ�գ�ת�Ƶĵ�����֮��Ϊ
��2����֪��101kpaʱ��CO�� ȼ����Ϊ283KJ/mol����ͬ�����£���2 molCH4ȼ������Һ̬ˮ�����ų�������Ϊ1mol CO��ȫȼ�շų�������6.30����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
ȼ����Ϊ283KJ/mol����ͬ�����£���2 molCH4ȼ������Һ̬ˮ�����ų�������Ϊ1mol CO��ȫȼ�շų�������6.30����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
��3��120�桢101Kpa�£�a ml �� CO��CH4��ɵĻ��������b ml O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
CO��CH4��ɵĻ��������b ml O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
�������������O2ǡ����ȫ��Ӧ������b ml CO2������������CH4���������Ϊ ������2λС����
����ȼ�պ����������С��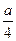 ml����a��b��ϵ����ѧ����ʽ��
ml����a��b��ϵ����ѧ����ʽ��
��1���������CO��CH4����ͬ�����·ֱ���ȫȼ�գ�ת�Ƶĵ�����֮��Ϊ
��2����֪��101kpaʱ��CO��
 ȼ����Ϊ283KJ/mol����ͬ�����£���2 molCH4ȼ������Һ̬ˮ�����ų�������Ϊ1mol CO��ȫȼ�շų�������6.30����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
ȼ����Ϊ283KJ/mol����ͬ�����£���2 molCH4ȼ������Һ̬ˮ�����ų�������Ϊ1mol CO��ȫȼ�շų�������6.30����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�� ��3��120�桢101Kpa�£�a ml ��
 CO��CH4��ɵĻ��������b ml O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
CO��CH4��ɵĻ��������b ml O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ���������������O2ǡ����ȫ��Ӧ������b ml CO2������������CH4���������Ϊ ������2λС����
����ȼ�պ����������С��
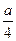 ml����a��b��ϵ����ѧ����ʽ��
ml����a��b��ϵ����ѧ����ʽ�� 
��

��ϰ��ϵ�д�
�����Ŀ



 ����� ����Ϊͬ���칹����� ��
����� ����Ϊͬ���칹����� �� (3)���� (4)���� (5)D (6)T
(3)���� (4)���� (5)D (6)T  (8)
(8) 
 (11)2,2����������
(11)2,2���������� ���� __________ ��������κ��Թ���������������ζ�����ʣ���Ӧ�Ļ�ѧ����ʽΪ _____���Ҵ������Ĺ����ŵ�����______
���� __________ ��������κ��Թ���������������ζ�����ʣ���Ӧ�Ļ�ѧ����ʽΪ _____���Ҵ������Ĺ����ŵ�����______