��Ŀ����
ij����ˮ�к�5.00��10-3mol��L-1�� ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
��Ϊ��������ˮ�����õ����Բ��� ��
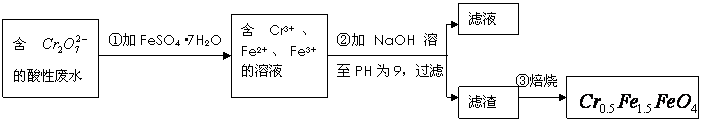
�� �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�


 ��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ��������ǣ�
��
��3���ڢ۲����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬���� ��
��4����ʹ1L�÷�ˮ�е� ��ȫת��Ϊ
��ȫת��Ϊ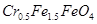 ����������Ҫ����
����������Ҫ����
��FeSO4��7H2O��
��14�֣���1��Cr2O72- + 6Fe2�� + 14H�� ��2Cr3+ + 6Fe3�� + 7H2O��4�֣�
��2����һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����ա���4�֣���3��Fe(OH)3��Fe(OH)2��2�֣���4��13.9��4�֣�
���������������1��Cr2O72-����ǿ�����ԣ��������������ӣ����Ը÷�Ӧ�����ӷ���ʽ��Cr2O72- + 6Fe2�� + 14H�� ��2Cr3+ + 6Fe3�� + 7H2O��
��2����PH��ֽ�ⶨ��ҺPH�IJ��������ǽ�һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����ա�
��3����Һ�к��������Ӻ��������ӣ����Լ�������������Һ��������Cr��OH��3�⣬����Fe(OH)3��Fe(OH)2��
��4��1L�÷�ˮ�е� �����ʵ�����5.00��10-3mol�������ԭ���غ��֪������
�����ʵ�����5.00��10-3mol�������ԭ���غ��֪������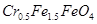 �����ʵ�����2.00��10-2mol�����Ը�����ԭ���غ��֪����ҪFeSO4��7H2O��������2.00��10-2mol��2.5��278g/mol��13.9g��
�����ʵ�����2.00��10-2mol�����Ը�����ԭ���غ��֪����ҪFeSO4��7H2O��������2.00��10-2mol��2.5��278g/mol��13.9g��
���㣺�������ӷ���ʽ����д��pH��ֽ������ҺpHֵ�IJ����Լ��йؼ���
�����������Դ�����ˮ��Cr2O72-Ϊ���壬�ص㿼��ѧ���Ļ�ѧʵ��������������Լ����ݴ����������������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԡ�����Ĺؼ�����ȷʵ��ԭ����Ȼ��������������ü��ɡ������ڽ����йؼ���ʱ��Ҫ���úø����غ㷨���������غ㡢ԭ���غ㡢���ӵĵ�ʧ�غ��Լ�����غ�ȡ�

 ��У����ϵ�д�
��У����ϵ�д�