��Ŀ����
���������ǿ��������������;�鲼������ҵ��
��1����ҵ�������̿���Ҫ�ɷ�ΪMnO2�������{����ط�Ϊ�������衣
�ٲ���һ�����̿�������Ĺ���KOH�Ϳ����ڸ����·�Ӧ����������أ�K2MnO4�����÷�Ӧ�Ļ�ѧ����ʽ��______________��
�ڲ������ʯīΪ��������Ϊ�������������أ�K2MnO4����Һ����_______������������������������õ�������ء�������Ӧ�Ļ�ѧ����ʽ��_______��
��2��ij�о�С���ù�ҵ����������ز����ķ����������ࣨ��K2MnO4��MnO2��Pb��Ca��Ԫ�أ�����ַ���Һ��ȡ��ҵ��̼���̣�MnCO3�������������£�

�����ַ�����Fe2+��������______________��
�ܲ���I������II������III��������_______��
����ҺC�г�����Ca2+��Mn2+��H+�⣬�����е���������_______��
��������C�в���CaCO3������ҺD�� <_______��
<_______��
[��֪��Ksp(MnCO3)=1��10��11��Ksp(CaCO3)=5��10��9]
��1����ҵ�������̿���Ҫ�ɷ�ΪMnO2�������{����ط�Ϊ�������衣
�ٲ���һ�����̿�������Ĺ���KOH�Ϳ����ڸ����·�Ӧ����������أ�K2MnO4�����÷�Ӧ�Ļ�ѧ����ʽ��______________��
�ڲ������ʯīΪ��������Ϊ�������������أ�K2MnO4����Һ����_______������������������������õ�������ء�������Ӧ�Ļ�ѧ����ʽ��_______��
��2��ij�о�С���ù�ҵ����������ز����ķ����������ࣨ��K2MnO4��MnO2��Pb��Ca��Ԫ�أ�����ַ���Һ��ȡ��ҵ��̼���̣�MnCO3�������������£�

�����ַ�����Fe2+��������______________��
�ܲ���I������II������III��������_______��
����ҺC�г�����Ca2+��Mn2+��H+�⣬�����е���������_______��
��������C�в���CaCO3������ҺD��
 <_______��
<_______��[��֪��Ksp(MnCO3)=1��10��11��Ksp(CaCO3)=5��10��9]
��16�֣���1���� 2MnO2+4KOH+O2  2K2MnO4+2H2O��3�֣�
2K2MnO4+2H2O��3�֣�
�� ��������2�֣� 2H2O+2e��=H2��+2OH����3�֣���2H+ + 2e��= H2��Ҳ��3�֣�
��2���� ���۵���Ԫ�ػ�ԭ��Mn2+��2�֣�
�� ���ˣ�2�֣�
�� K+��Na+��NH4+��2�֣�
�� 500��2�֣�
 2K2MnO4+2H2O��3�֣�
2K2MnO4+2H2O��3�֣��� ��������2�֣� 2H2O+2e��=H2��+2OH����3�֣���2H+ + 2e��= H2��Ҳ��3�֣�
��2���� ���۵���Ԫ�ػ�ԭ��Mn2+��2�֣�
�� ���ˣ�2�֣�
�� K+��Na+��NH4+��2�֣�
�� 500��2�֣�
�����������1���ٸ������⣬��Ԫ�صĻ��ϼ���+4����Ϊ+6���ɴ��ƶ�������Ϊ�����е����������ݻ��ϼ�����������ȡ�ԭ���غ��ƶϣ��÷�ӦʽΪ2MnO2+4KOH+O2
 2K2MnO4+2H2O������Ԫ�صĻ��ϼ���+6��Ϊ+7������������Ӧ�����ݵ��ԭ���ƶϣ��÷�Ӧ���������������������Һ�е�H+��K+���������������Խ�ǿ��H+������������ԭ��Ӧ����2H++2e��=H2����2H2O+2e��=H2��+2OH������2���ۢܢݸ��ݹ�������ͼ��Ŀ��������ƿ�֪����Ԫ�صĻ��ϼ���+6��Ϊ+2����Ҫ������ʵĻ�ԭ�������ַ�����Fe2+���Խ������е�MnO4����ԭΪMn2+��Fe2+��MnO4������ΪFe3+��������ҺpH��4ʱ��Fe3+��ȫ��ΪFe(OH)3�����������IΪ���ˣ���ҺA����Ҫ�ɷ�ΪMn2+��Fe2+��Pb2+��Ca2+��H+�ȣ�����A�ijɷ�ΪFe(OH)3��Na2S��Һ�ܳ�ȥFe2+��Pb2+��H+�ȣ��ֱ�����FeS��PbS������H2S����ȣ������IIΪ���ˣ���ҺB����Ҫ�ɷ�ΪMn2+��Ca2+��S2����Na+�ȣ�Na2S���Σ�ˮ�������Һ�ʼ��ԣ�NH4HCO3�ڼ��������±�ΪNH4+��CO32��������Ksp(MnCO3)<Ksp(CaCO3)��CO32����Mn2+��Ca2+���ʱ��������MnCO3�����������IIIΪ���ˣ��������ӹ����ƶϣ���ҺC����Ҫ������ΪMn2+��Ca2+��H+��Na+��NH4+���������⣬CaCO3(s)+Mn2+(aq)
2K2MnO4+2H2O������Ԫ�صĻ��ϼ���+6��Ϊ+7������������Ӧ�����ݵ��ԭ���ƶϣ��÷�Ӧ���������������������Һ�е�H+��K+���������������Խ�ǿ��H+������������ԭ��Ӧ����2H++2e��=H2����2H2O+2e��=H2��+2OH������2���ۢܢݸ��ݹ�������ͼ��Ŀ��������ƿ�֪����Ԫ�صĻ��ϼ���+6��Ϊ+2����Ҫ������ʵĻ�ԭ�������ַ�����Fe2+���Խ������е�MnO4����ԭΪMn2+��Fe2+��MnO4������ΪFe3+��������ҺpH��4ʱ��Fe3+��ȫ��ΪFe(OH)3�����������IΪ���ˣ���ҺA����Ҫ�ɷ�ΪMn2+��Fe2+��Pb2+��Ca2+��H+�ȣ�����A�ijɷ�ΪFe(OH)3��Na2S��Һ�ܳ�ȥFe2+��Pb2+��H+�ȣ��ֱ�����FeS��PbS������H2S����ȣ������IIΪ���ˣ���ҺB����Ҫ�ɷ�ΪMn2+��Ca2+��S2����Na+�ȣ�Na2S���Σ�ˮ�������Һ�ʼ��ԣ�NH4HCO3�ڼ��������±�ΪNH4+��CO32��������Ksp(MnCO3)<Ksp(CaCO3)��CO32����Mn2+��Ca2+���ʱ��������MnCO3�����������IIIΪ���ˣ��������ӹ����ƶϣ���ҺC����Ҫ������ΪMn2+��Ca2+��H+��Na+��NH4+���������⣬CaCO3(s)+Mn2+(aq) MnCO3(s)+Ca2+(aq)���÷�Ӧ��ƽ�ⳣ��K=
MnCO3(s)+Ca2+(aq)���÷�Ӧ��ƽ�ⳣ��K= =
=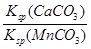 =
= =500��
=500��
��ϰ��ϵ�д�
�����Ŀ
 2ClO2����K2SO4��2CO2����2H2O������˵������ȷ����
2ClO2����K2SO4��2CO2����2H2O������˵������ȷ���� 2HNO3��9H2O��4N2����Ӧ�У�����ԭ�ĵ�ԭ���뱻�����ĵ�ԭ����Ŀ��Ϊ
2HNO3��9H2O��4N2����Ӧ�У�����ԭ�ĵ�ԭ���뱻�����ĵ�ԭ����Ŀ��Ϊ CO2��g��+H2��g�� �õ�������������
CO2��g��+H2��g�� �õ�������������