��Ŀ����
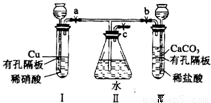
����ͼʵ��װ�ã�����a��b��cΪ���ɼС�
����1���������a��c���ر�b����I�з�Ӧ�����ӷ���ʽΪ________________________������2�������I���е�ϡ���ỻ��Ũ���ᣬ����a��c���ر�b����һ����۲죬���Կ���II�����ˮ�еĵ��ܿ�������ð���������������______ɫ��Һ��������Ϊ_____ɫ��
��3�������⣨1����ʵ���У�ҪʹII�������ʼ�ձ�����ɫ��Ӧ�ȹر�_____����a��b����ͬ��������____��c��ʹ������CO2�����ž�II��Ŀ������ٹر�_____��_____��
��4������32gͭ����ʢ��150mLһ��Ũ�������I��ʹ֮ǡ����ȫ��Ӧ��������NO2��NO��������ڱ�״���µ����Ϊ11.2L����NOΪ__________mol��ԭ������Һ��Ũ��Ϊ___________mol![]() L-1��
L-1��
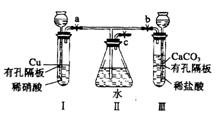
��1��3Cu+8H++2NO![]() ����=
����= ![]() ��2�֣�
��2�֣�
��2������ �ޣ�ÿ��1�֣���2�֣�
��3��a��b��b��a��ÿ��1�֣���4�֣�
��4��0.25 10��ÿ��2�֣���4�֣�

��ϰ��ϵ�д�
�����Ŀ
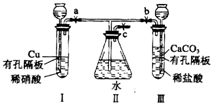 ����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�
����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�
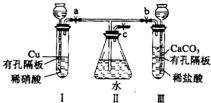 ����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�
����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�