��Ŀ����
ij��Ȼ��Ļ�ѧ��ɿ���ΪaNa2CO3��bNaHCO3��cH2O (a��b��cΪ������)��Ϊȷ������ɣ���ѧ��ȤС���ͬѧ����������ʵ�飺
(1)���Է�����
��ȡ������Ȼ����Ʒ�����Թ��У��þƾ��Ƽ��ȣ����Թܿ���Һ�����ɣ���Һ����ʹ��ˮ����ͭ�������ܷ�˵����Ʒ�к��ᾧˮ���Լ������ɡ�_________________________________��
���������һ����ʵ�鷽����ȷ����Ʒ�к���![]() ��
��
______________________________________________________________________________��
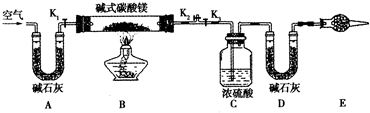
(2)������������С��ͬѧ�������ͼ��ʾװ�ã��ⶨ��Ȼ��Ļ�ѧ��ɡ�

ʵ�鲽�裺
�ٰ���ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����____________________��
A����ʯ�ҵ�������_______________��E����ʯ�ҵ�������__________________________��
�ڳ�ȡ��Ȼ����Ʒ7.3 g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿ������Ϊ87.6 g��װ��ʯ�ҵ�U�ι�D������Ϊ74.7 g��
�۴���K1��K2���ر�K3������������������ӡ�
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���K1������������������ӣ�Ȼ��Ƶ�װŨ�����ϴ��ƿ����Ϊ88.5 g��װ��ʯ�ҵ�U�ι�D������Ϊ75.8 g���ò����л���������������ӵ�Ŀ����_____________�������Ƶ�������Ȼ��Ļ�ѧʽΪ_____________��
(1)���Է�����
�ٲ��ܣ���ΪNaHCO3���ȷֽ�Ҳ�ܲ���ˮ����
��ȡ������Ʒ����ˮ���μ�CaCl2����BaCl2����Һ���а�ɫ�������ɣ�˵����CO2-3
(2)����������
�ټ��װ�õ������� ��ȥ�����е�CO2��H2O��g��
��ֹ������CO2��H2O(g)����Dװ�õ��²�����ȷ
�ݽ�װ�������ɵ�CO2��H2O��g��ȫ������C��Dװ���б����� Na2CO3��2NaHCO3��H2O
����:
���⿼��Na2CO3��NaHCO3�й����ʡ���������ӵļ��飬�ۺ��Խ�ǿ��(1)����2NaHCO3![]() Na2CO3+CO2��+H2O�����Բ���˵����Ʒ�к��ᾧˮ��Ca2++
Na2CO3+CO2��+H2O�����Բ���˵����Ʒ�к��ᾧˮ��Ca2++![]() ====CaCO3���ǽ���Ϥ��֪ʶ�㣬����
====CaCO3���ǽ���Ϥ��֪ʶ�㣬����![]() �Ĵ��ڣ�ע�ⲻ���ó���ʯ��ˮ�����������飬ԭ������
�Ĵ��ڣ�ע�ⲻ���ó���ʯ��ˮ�����������飬ԭ������![]() �ĸ��š�(2)�ڶ�������ʱ����������2NaHCO3
�ĸ��š�(2)�ڶ�������ʱ����������2NaHCO3![]() Na2CO3+CO2��+H2O ���ԭ�����������֪��װ��ʯ�ҵ�U�ι�D������������CO2����Ϊ75.8 g-74.7=1.1 g��n(CO2)��0.025 mol��
Na2CO3+CO2��+H2O ���ԭ�����������֪��װ��ʯ�ҵ�U�ι�D������������CO2����Ϊ75.8 g-74.7=1.1 g��n(CO2)��0.025 mol��
2NaHCO3![]() Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
2 1 1 1
0.05 mol0.025 mol0.025 mol0.025 mol
m��NaHCO3��=0.05 mol��84 g��mol-1=4.2 g
���Ծ�����![]() ��
��
m��H2O��=0.025 mol��18 g��mol-1=0.45 g
m(Na2CO3)��7.3 g-4.2 g-0.45 g��2.65 g��
![]() ���ɴ˿�֪������
���ɴ˿�֪������
n(Na2CO3)��n(NaHCO3)��n(H2O)��0.025 mol��0.05 mol��0.025 mol��1��2��1��
���Ծ��廯ѧʽΪNa2CO3��2NaHCO3��H2O��

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�