��Ŀ����
��֪��
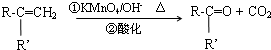
��R��R���ʾ����������ţ�
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5molA��ȫȼ��ֻ����3molCO2��2.5molH2O��A�ɷ�����ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ�����ɺ�������Ԫ��״������
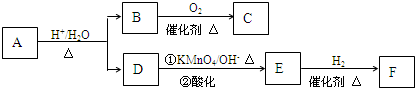
��ش�
��1��1molB�������Ľ����Ʒ�Ӧ������22.4L����״����H2��B�����������ŵ�������______��B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ��______��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������______��
��3��F�ɷ��������������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ��______��
����F������ʹ Br2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ��______��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ��______��
��4��A�Ľṹ��ʽ��______��
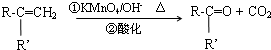
��R��R���ʾ����������ţ�
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5molA��ȫȼ��ֻ����3molCO2��2.5molH2O��A�ɷ�����ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ�����ɺ�������Ԫ��״������

��ش�
��1��1molB�������Ľ����Ʒ�Ӧ������22.4L����״����H2��B�����������ŵ�������______��B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ��______��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������______��
��3��F�ɷ��������������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ��______��
����F������ʹ Br2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ��______��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ��______��
��4��A�Ľṹ��ʽ��______��
���ƶ�A�ķ���ʽ����0.5molA��ȫȼ��ֻ����3molCO2��2.5molH2O����֪1molA�к�6molC��10molH����O��
=3����A�ķ���ʽΪC6H10O3��
��D�ķ���ʽΪC4H6O2����B�ķ���ʽΪC2H6O2����1molB���Ʒ�Ӧ���ɱ����22.4L��������BΪ��Ԫ������Ϊ�Ҷ�����HOCH2CH2OH��B������ΪC��
B��C����Է�������֮��Ϊ4����CΪ�Ҷ�ȩ���ṹ��ʽΪ��OHC-CHO����������F��Ӧ�����ɺ�������Ԫ��״��������֪F�Ľṹ��ʽΪ��
CH3CH��OH��COOH��E�Ľṹ��ʽΪ��CH3COCOOH����Dת��ΪE�������������Ϣ��֪D��̼̼˫����D�Ľṹ��ʽΪCH2=C��CH3��COOH����B��D�Ľṹ��ʽ
��Aˮ������B��D����֪A�Ľṹ��ʽΪ��CH2=C��CH3��COOCH2CH2OH��
��1��BΪ�Ҷ�������������Ϊ�ǻ����Ҷ���������Ϊ�Ҷ�ȩ����Ӧ����ʽΪ��HOCH2-CH2OH+O2
OHC-CHO+2H2O��
�ʴ�Ϊ���ǻ���HOCH2-CH2OH+O2
OHC-CHO+2H2O��
��2��D�Ľṹ��ʽΪ��CH2=C��CH3��COOH����������̼̼˫�����Ȼ�����D������ͬ�����ŵ�ͬ���칹��GΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��
�ʴ�Ϊ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��3����������F�γ���Ԫ�������ṹʽΪ��
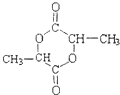
���ʴ�Ϊ��

��
����F������ʹ Br2��CCl4��Һ��ɫ���л���H����֪F��������ȥ��Ӧ����Ӧ����ʽΪ��
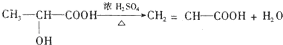
��
�ʴ�Ϊ��
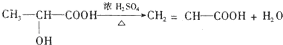
��
��F���ǻ����Ȼ����ɷ������۷�Ӧ���ɾ�������Ӧ����ʽΪ��
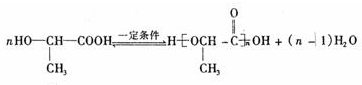
��
�ʴ�Ϊ��

��
��4���������ƶϿ�֪A�Ľṹ��ʽΪ��CH2=C��CH3��COOCH2CH2OH���ʴ�Ϊ��CH2=C��CH3��COOCH2CH2OH��
| 130-6��12-10��1 |
| 16 |
��D�ķ���ʽΪC4H6O2����B�ķ���ʽΪC2H6O2����1molB���Ʒ�Ӧ���ɱ����22.4L��������BΪ��Ԫ������Ϊ�Ҷ�����HOCH2CH2OH��B������ΪC��
B��C����Է�������֮��Ϊ4����CΪ�Ҷ�ȩ���ṹ��ʽΪ��OHC-CHO����������F��Ӧ�����ɺ�������Ԫ��״��������֪F�Ľṹ��ʽΪ��
CH3CH��OH��COOH��E�Ľṹ��ʽΪ��CH3COCOOH����Dת��ΪE�������������Ϣ��֪D��̼̼˫����D�Ľṹ��ʽΪCH2=C��CH3��COOH����B��D�Ľṹ��ʽ
��Aˮ������B��D����֪A�Ľṹ��ʽΪ��CH2=C��CH3��COOCH2CH2OH��
��1��BΪ�Ҷ�������������Ϊ�ǻ����Ҷ���������Ϊ�Ҷ�ȩ����Ӧ����ʽΪ��HOCH2-CH2OH+O2
| ���� |
| �� |
�ʴ�Ϊ���ǻ���HOCH2-CH2OH+O2
| ���� |
| �� |
��2��D�Ľṹ��ʽΪ��CH2=C��CH3��COOH����������̼̼˫�����Ȼ�����D������ͬ�����ŵ�ͬ���칹��GΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��
�ʴ�Ϊ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��3����������F�γ���Ԫ�������ṹʽΪ��

���ʴ�Ϊ��

��
����F������ʹ Br2��CCl4��Һ��ɫ���л���H����֪F��������ȥ��Ӧ����Ӧ����ʽΪ��
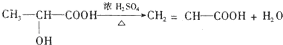
��
�ʴ�Ϊ��
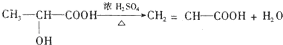
��
��F���ǻ����Ȼ����ɷ������۷�Ӧ���ɾ�������Ӧ����ʽΪ��

��
�ʴ�Ϊ��

��
��4���������ƶϿ�֪A�Ľṹ��ʽΪ��CH2=C��CH3��COOCH2CH2OH���ʴ�Ϊ��CH2=C��CH3��COOCH2CH2OH��

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ
 ��R��R���ʾ����������ţ�
��R��R���ʾ����������ţ�



 ���ϳɾ۷���E��·�ߣ�
���ϳɾ۷���E��·�ߣ�




 �ṹ
�ṹ

 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�
 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�