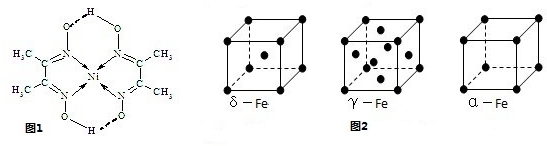
��Ŀ����
[��ѧ--ѡ���л���ѧ����]
ij������A��һ���������ܷ�������ͼ��ʾ��ת�䣺

����ֻ��B1����ʹ��ˮ��ɫ��������Na2CO3 ��Һ��Ӧ�ų�CO2���ش��������⣺
��1��ָ����Ӧ���ͣ�X______��Ӧ��Y______��Ӧ��
��2������A�Ľṹ��ʽΪ______��
��3��д��C1C2�Ļ�ѧ����ʽ______��
��4����F��Ϊͬ���칹�壨��������״�ṹ���Һ˴Ź�������ֻ��һ�ַ���л���Ľṹ��ʽ��______����дһ�֣�
��5����C2������ͬ�����ŵ�ͬ���칹�壨������C2��ͬ��̼ԭ���ϲ����ж���ǻ�������______�֣�
ij������A��һ���������ܷ�������ͼ��ʾ��ת�䣺

����ֻ��B1����ʹ��ˮ��ɫ��������Na2CO3 ��Һ��Ӧ�ų�CO2���ش��������⣺
��1��ָ����Ӧ���ͣ�X______��Ӧ��Y______��Ӧ��
��2������A�Ľṹ��ʽΪ______��
��3��д��C1C2�Ļ�ѧ����ʽ______��
��4����F��Ϊͬ���칹�壨��������״�ṹ���Һ˴Ź�������ֻ��һ�ַ���л���Ľṹ��ʽ��______����дһ�֣�
��5����C2������ͬ�����ŵ�ͬ���칹�壨������C2��ͬ��̼ԭ���ϲ����ж���ǻ�������______�֣�
ֻ�����������Ի��Ǽ��Ի�����ˮ����Եõ�������������ࣨ���������Σ�������ˮ�����B1����ʹ��ˮ��ɫ��������Na2CO3 ��Һ��Ӧ�ų�CO2��˵�����к���̼̼˫���Լ��Ȼ�������C1�Ǻ����ǻ�����ԭ�ӵ��л����ʣ��ڼ��Ի����£���ԭ��ˮ�����ɴ��ǻ�������C2��������Ϊ�����࣬����֪��C2���ڴ��࣬��F�Ľṹ��ʽ����֪��C2�Ľṹ��ʽΪ��OHCH2CH��CH3��CH2OH����C1�Ľṹ��ʽΪ��OHCH2CH��CH3��CH2Br������B1�Ľṹ��ʽΪ��

��A��B1��C1����������Ӧ�γɵ�������A�Ľṹ��ʽΪ��

��
��1��A�����Ի����»ᷢ��ˮ�ⷴӦ����X����ˮ�ⷴӦ����ȡ����Ӧ��ǿ��Ĵ���Һ�������ȴ���������ȥ��Ӧ���������ʴ�Ϊ��ȡ������ˮ�⣩����ȥ��
��2������A�Ľṹ��ʽΪ

���ʴ�Ϊ��

��
��3��C1ת��ΪC2�Ĺ��̣���±����ˮ��Ĺ��̣��仯ѧ����ʽΪ��

���ʴ�Ϊ��

��
��4����F��Ϊͬ���칹�壨��������״�ṹ�����Һ˴Ź�������ֻ��һ�ַ弴ֻ��һ�����͵ĵ�Ч��ԭ�ӣ������л���Ľṹ��ʽΪ��

���ʴ�Ϊ��

��
��5��C2�Ľṹ��ʽΪ��OHCH2CH��CH3��CH2OH����C2������ͬ�����ţ������������ǻ�����ͬ���칹����OHCH2CHOHCH2CH3��OHCH2CH2CH��OH��CH3��
OHCH2CH2CH2CH2OH��CH3CHOHCHOHCH3��OHCH2COH��CH3��CH3����5�֣��ʴ�Ϊ��5��

��A��B1��C1����������Ӧ�γɵ�������A�Ľṹ��ʽΪ��

��
��1��A�����Ի����»ᷢ��ˮ�ⷴӦ����X����ˮ�ⷴӦ����ȡ����Ӧ��ǿ��Ĵ���Һ�������ȴ���������ȥ��Ӧ���������ʴ�Ϊ��ȡ������ˮ�⣩����ȥ��
��2������A�Ľṹ��ʽΪ

���ʴ�Ϊ��

��
��3��C1ת��ΪC2�Ĺ��̣���±����ˮ��Ĺ��̣��仯ѧ����ʽΪ��

���ʴ�Ϊ��

��
��4����F��Ϊͬ���칹�壨��������״�ṹ�����Һ˴Ź�������ֻ��һ�ַ弴ֻ��һ�����͵ĵ�Ч��ԭ�ӣ������л���Ľṹ��ʽΪ��

���ʴ�Ϊ��

��
��5��C2�Ľṹ��ʽΪ��OHCH2CH��CH3��CH2OH����C2������ͬ�����ţ������������ǻ�����ͬ���칹����OHCH2CHOHCH2CH3��OHCH2CH2CH��OH��CH3��
OHCH2CH2CH2CH2OH��CH3CHOHCHOHCH3��OHCH2COH��CH3��CH3����5�֣��ʴ�Ϊ��5��

��ϰ��ϵ�д�
�����Ŀ




 [��ѧѡ��3�����ʽṹ������]
[��ѧѡ��3�����ʽṹ������]