��Ŀ����
 ��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��
��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��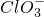 �����ֺ���Ԫ�ص����ӣ�����C1O-��
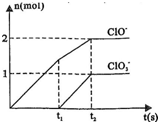
�����ֺ���Ԫ�ص����ӣ�����C1O-��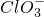 �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
��1��t1ǰ������������______ ���ѧʽ����
��2��t2ʱ��Ca��OH��2��Cl2������Ӧ���ܵ����ӷ���ʽΪ��______��
��3����ʯ�����к���Ca��OH��2�����ʵ�����______mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ��������______������ĸ����
A��NaCl��Cl2 B��NaCl��NaClO�� C��NaClO3��NaClO4 D��NaCl��NaClO3
��5����ƽ�������ӷ���ʽ��
______Fe��OH��3+______ClO-+______OH-----______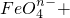 ______Cl-+______H2O��
______Cl-+______H2O��
�⣺��1��������ԭ��Ӧ�ڵ����������ǻ�ԭ�����������ɵ����ʣ����ϼ��ڱ仯�����ߣ�����Ԫ�ػ��ϼ��������ɵIJ�����ͼ�������t1ǰ����������ֻ��Ca��ClO��2���ʴ�Ϊ��Ca��ClO��2��
��2��t2ʱ��Ca��OH��2��Cl2������Ӧ������ͼ�������֪���ɴ��������������������ʵ���֮��Ϊ2��1�������������������ӷ���ʽ����дԭ�����غ㡢ԭ���غ���ƽ����ʽ��
5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O��
�ʴ�Ϊ��5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O��
��3��t2ʱ���������ƺ�����ǡ�÷�Ӧ�����ݷ�Ӧ�����ӷ���ʽ��֪��
5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O�����������������ʵ���Ϊ��5mol���ʴ�Ϊ��5mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը������������ԭ��Ӧ����Ԫ�ػ��ϼ۴�+3�����������䱬ը��IJ����� ����Ԫ�ػ��ϼ��д���+3�ۺ�С��+3�۵Ļ����
A������Ԫ�ػ��ϼ�Ϊ-1��0�ۣ������ϣ�
B����Ԫ�ػ��ϼ�-1��+1�ۣ������ϣ�
C����Ԫ�ػ��ϼ�Ϊ+5��+7�ۣ������ϣ�
D����Ԫ�ػ��ϼ�Ϊ-1��+5�ۣ����ϣ�
�ʴ�Ϊ��D
��5�����ݻ��ϼ۱仯��ClO-��Cl-��2e-��Fe��OH��3��FeO4n-����5-n��e-������ת������2��5-n����������ӷ���ʽ�ĵ����غ㡢����غ㡢ԭ���غ���з�����ƽ��д�����ӷ���ʽΪ��
2Fe��OH��3+��5-n��ClO-+2nOH-=2FeO4n-+��5-n��Cl-+��3+n��H2O
�ʴ�Ϊ��2����5-n����2n��2����5-n������n+3����
��������1��������ԭ��Ӧ�ڵ����������ǻ�ԭ�����������ɵ����ʣ����ϼ��ڱ仯�����ߣ�
��2��t2ʱ��Ca��OH��2��Cl2������Ӧ������ͼ�������֪���ɴ��������������������ʵ���֮��Ϊ2��1�������������������ӷ���ʽ����дԭ�����غ㡢ԭ���غ���ƽ����ʽ��ע��ʯ������������Щ��ѧʽ��
��3����ʯ�����к���Ca��OH��2�����ʵ�������ͼ������ӷ���ʽ���㣬t2ʱ����������������ǡ��ȫ����Ӧ�����������������ʵ���Ϊ5mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը������������ԭ��Ӧ����Ԫ�ػ��ϼ۴�+3�����������䱬ը��IJ����� ����Ԫ�ػ��ϼ��д���+3�ۺ�С��+3�۵Ļ����
��5������������ԭ��Ӧ�Ļ��ϼ�����������ͬ��������ƽ�����ԭ���غ㡢����غ㣻
���������⿼������������Ӧ�ã��������������Ʒ�Ӧ��ͼ������жϣ����ӷ���ʽ����д������������ԭ��Ӧ�ĵ����غ㷽����ƽ���ӷ���ʽ��������ԭ��Ӧ�Ļ��ϼ۱仯����Ӧ�ã���Ŀ�Ѷ��еȣ�
��2��t2ʱ��Ca��OH��2��Cl2������Ӧ������ͼ�������֪���ɴ��������������������ʵ���֮��Ϊ2��1�������������������ӷ���ʽ����дԭ�����غ㡢ԭ���غ���ƽ����ʽ��
5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O��
�ʴ�Ϊ��5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O��
��3��t2ʱ���������ƺ�����ǡ�÷�Ӧ�����ݷ�Ӧ�����ӷ���ʽ��֪��
5Ca��OH��2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O�����������������ʵ���Ϊ��5mol���ʴ�Ϊ��5mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը������������ԭ��Ӧ����Ԫ�ػ��ϼ۴�+3�����������䱬ը��IJ����� ����Ԫ�ػ��ϼ��д���+3�ۺ�С��+3�۵Ļ����
A������Ԫ�ػ��ϼ�Ϊ-1��0�ۣ������ϣ�
B����Ԫ�ػ��ϼ�-1��+1�ۣ������ϣ�
C����Ԫ�ػ��ϼ�Ϊ+5��+7�ۣ������ϣ�
D����Ԫ�ػ��ϼ�Ϊ-1��+5�ۣ����ϣ�
�ʴ�Ϊ��D
��5�����ݻ��ϼ۱仯��ClO-��Cl-��2e-��Fe��OH��3��FeO4n-����5-n��e-������ת������2��5-n����������ӷ���ʽ�ĵ����غ㡢����غ㡢ԭ���غ���з�����ƽ��д�����ӷ���ʽΪ��
2Fe��OH��3+��5-n��ClO-+2nOH-=2FeO4n-+��5-n��Cl-+��3+n��H2O
�ʴ�Ϊ��2����5-n����2n��2����5-n������n+3����
��������1��������ԭ��Ӧ�ڵ����������ǻ�ԭ�����������ɵ����ʣ����ϼ��ڱ仯�����ߣ�
��2��t2ʱ��Ca��OH��2��Cl2������Ӧ������ͼ�������֪���ɴ��������������������ʵ���֮��Ϊ2��1�������������������ӷ���ʽ����дԭ�����غ㡢ԭ���غ���ƽ����ʽ��ע��ʯ������������Щ��ѧʽ��
��3����ʯ�����к���Ca��OH��2�����ʵ�������ͼ������ӷ���ʽ���㣬t2ʱ����������������ǡ��ȫ����Ӧ�����������������ʵ���Ϊ5mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը������������ԭ��Ӧ����Ԫ�ػ��ϼ۴�+3�����������䱬ը��IJ����� ����Ԫ�ػ��ϼ��д���+3�ۺ�С��+3�۵Ļ����
��5������������ԭ��Ӧ�Ļ��ϼ�����������ͬ��������ƽ�����ԭ���غ㡢����غ㣻
���������⿼������������Ӧ�ã��������������Ʒ�Ӧ��ͼ������жϣ����ӷ���ʽ����д������������ԭ��Ӧ�ĵ����غ㷽����ƽ���ӷ���ʽ��������ԭ��Ӧ�Ļ��ϼ۱仯����Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��
��2013?����ģ�⣩��֪Ca��OH��2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl-��ClO-��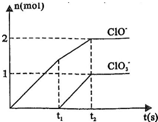
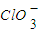 �����ֺ���Ԫ�ص����ӣ�����C1O-��
�����ֺ���Ԫ�ص����ӣ�����C1O-�� �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ�� ______Cl-+______H2O��
______Cl-+______H2O��