��Ŀ����
��2008?�ع�һģ����֪�������ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ�����������ˮ��Ӧ��
 +H2O
+H2O
���з���ʽΪC9H8O2Br2������M���л���C����Է�������Ϊ60����һ�������¿ɷ�������һϵ�з�Ӧ��

��ش��������⣺
��1��G��H�ķ�Ӧ������
��2��C�Ľṹ��ʽΪ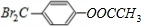
 ��
��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ����A��B�Ļ�ѧ����ʽ��
 ��
��

| �Զ���ˮ |
 +H2O
+H2O���з���ʽΪC9H8O2Br2������M���л���C����Է�������Ϊ60����һ�������¿ɷ�������һϵ�з�Ӧ��

��ش��������⣺
��1��G��H�ķ�Ӧ������
�ӳɷ�Ӧ
�ӳɷ�Ӧ
����2��C�Ľṹ��ʽΪ
CH3COOH
CH3COOH
��M�Ľṹ��ʽΪ

��3��д�����з�Ӧ�Ļ�ѧ����ʽ����A��B�Ļ�ѧ����ʽ��
CH3CHO+2Ag��NH3��2OH
2Ag��+CH3COONH4+3NH3+H2O
| ||
CH3CHO+2Ag��NH3��2OH
2Ag��+CH3COONH4+3NH3+H2O
| ||
ɾ���˿�
ɾ���˿�
����H��I�Ļ�ѧ����ʽ��

������C����Է�������Ϊ60����A
B��C����AΪȩ��BΪ�������Σ�CΪ���ᣬ����CΪCH3COOH��BΪCH3CHOONH4��AΪCH3CHO��
E��������ͭ��Һ��Ӧ����ש��ɫ������F��˵��E�к���-CHO��G���Ȼ�����Һ��ɫ��G�к��з��ǻ�-OH��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����M��Ũ���ᡢ��������������������D�������Ϣ��֪��DӦΪ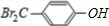 ��MΪ
��MΪ ����EΪ
����EΪ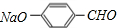 �������л����ת����֪FΪ
�������л����ת����֪FΪ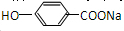 ��GΪ
��GΪ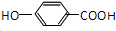 ��HΪ
��HΪ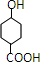 ����IΪ
����IΪ ������л���Ľṹ�����ʿɽ����⣮
������л���Ľṹ�����ʿɽ����⣮
| ����������Һ |
E��������ͭ��Һ��Ӧ����ש��ɫ������F��˵��E�к���-CHO��G���Ȼ�����Һ��ɫ��G�к��з��ǻ�-OH��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����M��Ũ���ᡢ��������������������D�������Ϣ��֪��DӦΪ
 ��MΪ
��MΪ ����EΪ
����EΪ �������л����ת����֪FΪ
�������л����ת����֪FΪ ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ������л���Ľṹ�����ʿɽ����⣮
������л���Ľṹ�����ʿɽ����⣮����⣺C����Է�������Ϊ60����A
B��C����AΪȩ��BΪ�������Σ�CΪ���ᣬ����CΪCH3COOH��BΪCH3CHOONH4��AΪCH3CHO��
E��������ͭ��Һ��Ӧ����ש��ɫ������F��˵��E�к���-CHO��G���Ȼ�����Һ��ɫ��G�к��з��ǻ�-OH��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����M��Ũ���ᡢ��������������������D�������Ϣ��֪��DӦΪ ��MΪ
��MΪ ����EΪ
����EΪ �������л����ת����֪FΪ
�������л����ת����֪FΪ ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ��
��
��1��G��H�� �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ���� ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��
��2��������������֪��CΪCH3COOH��M�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ��CH3COOH�� ��
��
��3������A��B����ȩ��������Һ��������Ӧ����ʽΪ��CH3CHO+2Ag��NH3��2OH
2Ag��+CH3COONH4+3NH3+H2O��
��HΪ ��ͨ����ȥ��Ӧ������
��ͨ����ȥ��Ӧ������ ����Ӧ�ķ���ʽΪ��
����Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
2Ag��+CH3COONH4+3NH3+H2O�� ��
��
| ����������Һ |
E��������ͭ��Һ��Ӧ����ש��ɫ������F��˵��E�к���-CHO��G���Ȼ�����Һ��ɫ��G�к��з��ǻ�-OH��Hת��ΪIʱ������ֻ��һ�ֽṹ��˵��D��E��F��H��I�б����ϵ�ȡ�������ڶ�λ����M��Ũ���ᡢ��������������������D�������Ϣ��֪��DӦΪ
 ��MΪ
��MΪ ����EΪ
����EΪ �������л����ת����֪FΪ
�������л����ת����֪FΪ ��GΪ
��GΪ ��HΪ
��HΪ ����IΪ
����IΪ ��
����1��G��H��
 �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ���� ��
���ʴ�Ϊ���ӳɷ�Ӧ��
��2��������������֪��CΪCH3COOH��M�Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��CH3COOH��
 ��
����3������A��B����ȩ��������Һ��������Ӧ����ʽΪ��CH3CHO+2Ag��NH3��2OH
| ||
��HΪ
 ��ͨ����ȥ��Ӧ������
��ͨ����ȥ��Ӧ������ ����Ӧ�ķ���ʽΪ��
����Ӧ�ķ���ʽΪ�� ��
���ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
| ||
 ��
�����������⿼���л�����ƶϣ��漰����ȩ�����ᡢ±�����������ӵ����ʵȣ���Ŀ�Ѷ��еȣ�����ע����������Ϣ����ȡ���Ʒ������Ʒ����ϵķ����ƶϣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2008?�ع�һģ����ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ�����������й�˵����ȷ���ǣ�������
��2008?�ع�һģ����ͼ����ע�����м�������Na2SO3���壬����������Ũ���ᣨ�Բ��Ӵ�ֽ��Ϊ�����������й�˵����ȷ���ǣ�������