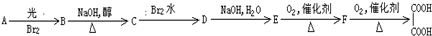��Ŀ����
��д���пհף�
��1��34g NH3����
��2���ڱ�״���£�35.5g Cl2�����Լ��
��3��������500mL 0.2mol/L Na2CO3��Һ����Ҫ����ƽ����Na2CO3?10H2O��������Ϊ
��1��34g NH3����
8
8
molԭ�ӣ�0.1molH2S��Լ��1.204��1023
1.204��1023
����ԭ�ӣ�ͬ��ͬѹ�£��������NH3��H2S�����������Ϊ1��2
1��2
�����еķ�����Ŀ֮��Ϊ1��1
1��1
����������NH3��H2S�з�����Ŀ֮��Ϊ2��1
2��1
����2���ڱ�״���£�35.5g Cl2�����Լ��
11.2
11.2
L��������������ȫ���������Ȼ��������������ʵ�����0.5
0.5
mol�������ɵ��Ȼ�����������1000gˮ�У��õ��ܶ�Ϊa g?cm-3�����ᣬ�����������ʵ���Ũ���ǣ�a/1.0365
1.0365
��mol/L����3��������500mL 0.2mol/L Na2CO3��Һ����Ҫ����ƽ����Na2CO3?10H2O��������Ϊ
28.6g
28.6g
��������õ�������Һ��ȡ��50mL��һ�Լ�ƿ�У���Ҫ�������ϱ�ǩ����ǩ�ϵ�������0.2mol/L Na2CO3��Һ
0.2mol/L Na2CO3��Һ
�����ٴ���ȡ��10mL��Һ��ˮϡ����20mL�������Һ��Na+�����ʵ���Ũ��Ϊ0.2mol/L
0.2mol/L
����������1�����ݰ�����������������������ʵ��������ݰ���٤������������Ȼ����е���ԭ�ӣ�������ͬ�����������ͬ���ʵ�����ͬ���������������������ȼ�������֮�ȣ�������ͬ�����ʵ�����Ħ�������ɷ��ȣ�
��2����������Ħ������������������������������������ķ�Ӧ��������������ʵ���������������ܶȼ������������ʵ���Ũ�ȣ�
��3����������һ�����ʵ���Ũ�ȵ���Һ������ɣ�������Һ�����ʽ����жϣ�
��2����������Ħ������������������������������������ķ�Ӧ��������������ʵ���������������ܶȼ������������ʵ���Ũ�ȣ�
��3����������һ�����ʵ���Ũ�ȵ���Һ������ɣ�������Һ�����ʽ����жϣ�
����⣨1�����������ʵ���Ϊ
=2mol������2mol��4=8molԭ�ӣ�0.1molH2S������0.2mol��ԭ�ӣ�������ԭ�ӵ���ĿΪ6.02��1023��0.2=1.204��1023��ͬ��ͬѹ�£��������NH3��H2S���壬������ͬ�����ʵ����������Ⱦ͵������ǵ�Ħ������֮�ȣ�������Ϊ17��34=1��2�����ʵ����ȵ��ڷ�����֮�ȣ���������Ϊ1��1��
�������İ������Ȼ��⣬������Ŀ֮����Ħ�������ɷ��ȣ�������֮��Ϊ34��17=2��1��
�ʴ�Ϊ��8��1.204��1023��1��2��1��1��2��1��
��2������£�35.5g���������ʵ���Ϊ0.5mol�����Ϊ11.2L����������Ӧ�����Ȼ��⣬��Ҫ�����������ʵ���Ϊ0.5mol��0.5mol������Ӧ����1mol�Ȼ��⣬�Ȼ��������Ϊ36.5����Һ�����Ϊ��
���Ȼ�����Һ��Ũ��Ϊ��
=
mol/L��
�ʴ�Ϊ��11.2��0.5��1.0365��
��3������500mL 0.2mol/L Na2CO3��Һ����Ҫ����ƽ����Na2CO3?10H2O��������ʵ���Ϊ��0.2mol/L��0.5L=0.1mol������Ϊ286g/mol��0.1mol=28.6g����ǩ�ϵ�����Ϊ0.2mol/L Na2CO3��Һ�����ٴ���ȡ��10mL��Һ��ˮϡ����20mL��̼���Ƶ�Ũ��Ϊ0.1mol/L����Һ��Na+�����ʵ���Ũ��Ϊ0.2mol/L��
�ʴ�Ϊ��28.6 g��0.2mol/L Na2CO3��Һ�� 0.2mol/L��
| 34g |
| 17g/mol |
�������İ������Ȼ��⣬������Ŀ֮����Ħ�������ɷ��ȣ�������֮��Ϊ34��17=2��1��
�ʴ�Ϊ��8��1.204��1023��1��2��1��1��2��1��
��2������£�35.5g���������ʵ���Ϊ0.5mol�����Ϊ11.2L����������Ӧ�����Ȼ��⣬��Ҫ�����������ʵ���Ϊ0.5mol��0.5mol������Ӧ����1mol�Ȼ��⣬�Ȼ��������Ϊ36.5����Һ�����Ϊ��
| 1000g+36.5g |
| 1000ag/L |
| 1mol | ||
|
| a |
| 1.0365 |
�ʴ�Ϊ��11.2��0.5��1.0365��
��3������500mL 0.2mol/L Na2CO3��Һ����Ҫ����ƽ����Na2CO3?10H2O��������ʵ���Ϊ��0.2mol/L��0.5L=0.1mol������Ϊ286g/mol��0.1mol=28.6g����ǩ�ϵ�����Ϊ0.2mol/L Na2CO3��Һ�����ٴ���ȡ��10mL��Һ��ˮϡ����20mL��̼���Ƶ�Ũ��Ϊ0.1mol/L����Һ��Na+�����ʵ���Ũ��Ϊ0.2mol/L��
�ʴ�Ϊ��28.6 g��0.2mol/L Na2CO3��Һ�� 0.2mol/L��
���������⿼���˰���٤���������й����ʵ����ļ��㡢һ�����ʵ���Ũ�ȵ���Һ�����ƣ�ע���˻���֪ʶ�Ŀ��飬�����ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ




 +Br2
+Br2 +HBr
+HBr