��Ŀ����
��16�֣���֪A��B��C��D��E��G��H��I��Ϊ���壬JΪ������Һ̬���ʣ�A��B��C��I��MΪ���ʣ���MΪ���ý�����G��H����ʱ�������̣����Ǵ������µ�ת����ϵ��ͼ�в��ַ�Ӧ��������ʡ�ԣ�����ش��й����⣺
��1��A���ӵĵ���ʽ�� ��G���ӵĿռ乹��Ϊ ��
��2�������£�pHֵ��Ϊ5��H��Һ��K��Һ����ˮ�����c(H+)֮��Ϊ ��
��3������X��ˮ��Һ��ͨ��G�������������� ��N��X�ж���M��Ԫ�أ��仯�ϼ��Ƿ���ͬ ��
��4��д��X+C��Y�����ӷ���ʽ ��
M����̬J�ڸ���ʱ��Ӧ�Ļ�ѧ����ʽ�� ��
��5���������������磬����ͬ����AԪ�ص�Z��Kʩ�õ������Ч�����õ��� - ���ѧʽ����
��6����ͨ��״���£���1 g B������C������ȼ������H����ʱ�ų�92.3 kJ��������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ ��
��16�֣�(1)��N![]() N�ã�1�֣� �����ͣ�1�֣� ��2��1*10��4��2�֣���
N�ã�1�֣� �����ͣ�1�֣� ��2��1*10��4��2�֣���
��3���Ȳ�����ɫ��������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��2�֣���һ����ͬ��2�֣�
��4��2Fe2+��Cl2=2Cl����2Fe3+��2�֣� 3Fe��4H2OFe3O4��4H2 ��2�֣�
��5��NH4Cl��2�֣� ![]() (6)2HCl(g)=H��(g)+Cl��(g)����H= +184.6 kJ��mol-1?��2�֣�
(6)2HCl(g)=H��(g)+Cl��(g)����H= +184.6 kJ��mol-1?��2�֣�
����:

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
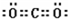
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�