��Ŀ����
12��ij��ѧС��Ϊ�ⶨ�ӵ�����KIO3����������������������ʵ�飮��֪��KIO3+5KI+3H2SO4�T3K2SO4+3I2+3H2O
I2+2Na2S2O3�TNa2S4O6+2NaI
����һ��ȷ��ȡa g�ӵ��Σ����Ƴ�250mL��Һ��
�������ȡ��������Һ25.00mL����ƿ�У���ϡ�����ữ���ټ���������KI��Һ��
����������bmol•L-1 Na2S2O3��Һ����Һ�ζ������������Һ���յ㣬��¼���ݣ����ظ��ζ�2�Σ�ƽ������Na2S2O3��Һ�����Ϊ12.00mL��
��1������һ������250mL��Һ�����õ��IJ����������ձ����������ͽ�ͷ�ι��⣬����250mL����ƿ��
��2����������ѡ�������Ϊָʾ��������ζ��յ�ʱ������Ϊ���������һ����Na2S2O3��Һʱ����Һ����ɫ��ȥ���Ұ��������Һ���ָ���ɫ��
��3��ʵ���ô˼ӵ�����KIO3����������=$\frac{4.28c}{a}$��100%��KIO3����Է�������Ϊ214����
��4���������У��ζ�ǰ�ζ���û����Na2S2O3��Һ��ϴ����ʹ���ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
���� ��1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������
��2�������ɵⵥ�ʲμӵķ�Ӧѡ��ʹ�õ�ָʾ�������ݷ�Ӧǰ��Һ��ɫ����Ӧ����Һ�жϵζ��յ㣻
��3�����ݷ�Ӧ�ҳ���Ӧ��ϵʽ��Ȼ������������ݼ����������KIO3������������
��4�����ݵζ���û����ϴ���´�װҺŨ��ƫ�ͣ�
��� �⣺��1������250mL��Һ�õ���������������ƽ��ҩ�ס�����������ͷ�ιܡ��ձ���250mL����ƿ����Ͳ�����ÿɲ��ã�����ͷ�ιܣ��ʻ���Ҫ�IJ��������У�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
��2�������������I2������ʹ�õ�����Ϊָʾ����������ۣ���Һ����ɫ����Na2S2O3��Һ�ζ���I2��Ӧ��ϣ���Һ��ɫ��ɫ���ζ��յ�����Ϊ����Һ��ɫ��ɫ��������ڲ��ָ���ɫ��
�ʴ�Ϊ�����������һ����Na2S2O3��Һʱ����Һ����ɫ��ȥ���Ұ��������Һ���ָ���ɫ��
��3��25mL��Һ����Na2S2O3�����ʵ���Ϊc mol•L-1��0.012L=0.012mol����250mL��ҺӦ����Na2S2O3�����ʵ���Ϊ0.012cmol��10=0.12cmol����250mL��Һ��KIO3�����ʵ���Ϊxmol����
KIO3��3I2��6Na2S2O3
1 6
xmol 0.12cmol
����x=0.02c
�ʼӵ�����KIO3����������$\frac{0.02cmol��214g/mol}{ag}$��100%=$\frac{4.28c}{a}$��100%��
�ʴ�Ϊ��$\frac{4.28c}{a}$��100%��
��4���ζ�ǰ�ζ���û����Na2S2O3��Һ��ϴ�����µζ����������ĵ�Na2S2O3��Һ���ƫ�ⶨ�ĵ���ص����ʵ���ƫ�ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼��������ԭ�ζ���Ӧ�á���ʵ��ԭ��������ʵ��װ�õ����ۡ��Ķ���ȡ��Ϣ���������Ѷ��еȣ��Ƕ�֪ʶ���ۺ����������������������Ŀ��飬ע�����֪ʶ�����գ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| A�� | O2�ڵ缫b�Ϸ�����ԭ��Ӧ | |
| B�� | ��Һ��OH-��缫b�ƶ� | |
| C�� | ��Ӧ���ĵ�NH3��O2�����ʵ���֮��Ϊ4��3 | |
| D�� | �����ĵ缫��ӦʽΪ��2NH3-6e-+6OH-�TN2+6H2O |
| A�� | Al | B�� | Mg | ||
| C�� | Na | D�� | ���߲���������һ���� |
| A�� | ��������Ԫ�ص����Ӱ뾶���������μ�С | |
| B�� | HCl��PCl5��N2��CO2����������ԭ�Ӷ����������8���ӵĽṹ | |
| C�� | �����ڿ��Բ����ڻ�ѧ����Ҳ����ͬʱ�������Ӽ����ۼ� | |
| D�� | ��ij���ӻ�����X2Y3��X3+��Y2-�ĵ��Ӳ�ṹ��ͬ����X��Y��Ԫ�ص�ԭ������֮�������5��15��29 |
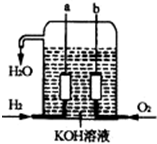 ��������������ɴ���ʹ�õ�����ȼ�ϵ����һ�����͵Ļ�ѧ��أ��乹����ͼ��ʾ�������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮
��������������ɴ���ʹ�õ�����ȼ�ϵ����һ�����͵Ļ�ѧ��أ��乹����ͼ��ʾ�������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮

