��Ŀ����
������й���ļ������Σ���������������ظ���λ����Ϊ��������ѧ�̲���NaCl����ṹͼ��ΪNaCl��һ����������֪FexO����ľ����ṹΪNaCl�ͣ����ھ���ȱ�ݣ�x��1��ʵ�ʲ��FexO������ܶȦ�=5.71 g��cm-3�������߳�d=4.28��10-10 m��(1)��FexO��x�ľ�����ֵ��x=__________��
(2)������FeԪ��ֻ��+2�ۺ�+3�ۣ���![]() =__________��
=__________��
(3)�����У���O2-�����������ȵ�Fe2+(��Fe3+)��Χ�ɵĿռ伸�ι�����_________��
A.������ B.�������� C.�������� D.������
(4)�����У�FeԪ�ص����Ӽ��������r=_________m��
������(1)���ݦ�= ����ľ����У��й�����Ϊ��MΪFexO��Ħ��������Na=6.02��1023 mol-1��n=4��V=(4.28��10-8)3 cm3����
����ľ����У��й�����Ϊ��MΪFexO��Ħ��������Na=6.02��1023 mol-1��n=4��V=(4.28��10-8)3 cm3����
(2)��Fe2+Ϊa mol��Fe3+Ϊb mol��O2-Ϊ1 mol����
![]()
![]()
��N![]() ��
��
(3)����ͼ��ʾ����һ����������ṹ��
![]()

�𰸣�(1)0.92 (2)![]() ��
��![]()
(3)C (4)3.02��10-10

��ϰ��ϵ�д�
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
�����Ŀ


 2s
2s 3s
3s 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ�� 
 2s
2s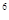 3s
3s 4s
4s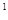 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��