��Ŀ����
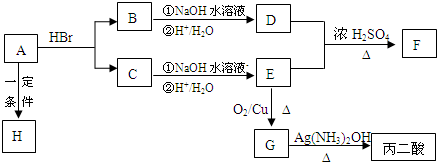
������A��B��C��D��E��F��G��H�������ᣨHOOCCH2COOH����ת����Ӧ�Ĺ�ϵͼ��

A��һ����״���ᣬ����ʽΪC3H4O2��F�к������߸�ԭ�ӹ��ɵĻ���H��һ�ָ߷��ӻ����
����д���пհף�
��1��C�Ľṹ��ʽ��__________ ��F�Ľṹ��ʽ��____________ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�ͷ�Ӧ���ͣ�ÿ������ʽ2�֣���Ӧ����1�֣���
�� A��H�Ļ�ѧ����ʽ��______________ ��____________ ��
�� B��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________________ ��________________ ��
�� G��������Ļ�ѧ����ʽ��_________________��____________ ��
��3��ʵ�����Ʊ�������Һ�IJ����ؼ��ǣ�________________��
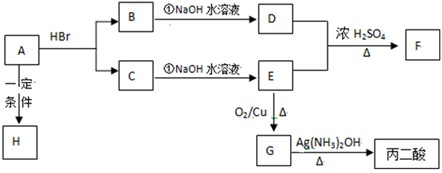
����д���пհף�
��1��C�Ľṹ��ʽ��__________ ��F�Ľṹ��ʽ��____________ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�ͷ�Ӧ���ͣ�ÿ������ʽ2�֣���Ӧ����1�֣���
�� A��H�Ļ�ѧ����ʽ��______________ ��____________ ��
�� B��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��____________________ ��________________ ��
�� G��������Ļ�ѧ����ʽ��_________________��____________ ��
��3��ʵ�����Ʊ�������Һ�IJ����ؼ��ǣ�________________��
��1��C: BrCH2CH2COOH �� F: 
��2�� �� ���Ӿ۷�Ӧ
���Ӿ۷�Ӧ
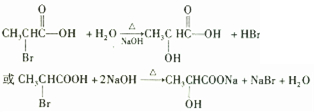
�� CH3CHBrCOOH + 2NaOH NaBr + H2O + CH3CH(OH)COONa ��ȡ����Ӧ
NaBr + H2O + CH3CH(OH)COONa ��ȡ����Ӧ
�� OHCCH2COOH + 2 Ag(NH3)2OH 2Ag + 2NH3��+ NH4OOCCH2COONH4 ��������Ӧ
2Ag + 2NH3��+ NH4OOCCH2COONH4 ��������Ӧ
��3������������Һ�еμӰ�ˮ������ǡ����ʧΪֹ

��2�� ��
 ���Ӿ۷�Ӧ
���Ӿ۷�Ӧ �� CH3CHBrCOOH + 2NaOH
 NaBr + H2O + CH3CH(OH)COONa ��ȡ����Ӧ
NaBr + H2O + CH3CH(OH)COONa ��ȡ����Ӧ�� OHCCH2COOH + 2 Ag(NH3)2OH
 2Ag + 2NH3��+ NH4OOCCH2COONH4 ��������Ӧ
2Ag + 2NH3��+ NH4OOCCH2COONH4 ��������Ӧ ��3������������Һ�еμӰ�ˮ������ǡ����ʧΪֹ

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
������A��B��C��D��E����Ԫ�ص�ijЩ���ʣ�
��1��Ԫ��A���γ��л������ҪԪ�أ����з����к���sp��sp3 �ӻ���ʽ����
A�� B��CH4 C��CH2=CHCH3D��CH3CH2C��CH E��CH3CH3
B��CH4 C��CH2=CHCH3D��CH3CH2C��CH E��CH3CH3
��2���������ʾʽд��E���⻯��ˮ��Һ�д��ڵ�������� ��
��3����ͬ�����£�AD2��BD2����������ˮ�е��ܽ�Ƚϴ���� ��д����ʽ���������� ��
��4����ͼ��ʾij��Ԫ��D��п�γɵĻ����ᄃ��������Zn��Dͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ ���û�����ľ����۵�ȸɱ��ߵö࣬ԭ���� ��

| A | B | C | D | E | |
| ���ϼ� | -4 | -2 | -1 | -2 | -1 |
| �縺�� | 2.55 | 2.58 | 3.16 | 3.44 | 3.98 |
A��
 B��CH4 C��CH2=CHCH3D��CH3CH2C��CH E��CH3CH3
B��CH4 C��CH2=CHCH3D��CH3CH2C��CH E��CH3CH3��2���������ʾʽд��E���⻯��ˮ��Һ�д��ڵ��������
��3����ͬ�����£�AD2��BD2����������ˮ�е��ܽ�Ƚϴ����
��4����ͼ��ʾij��Ԫ��D��п�γɵĻ����ᄃ��������Zn��Dͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ

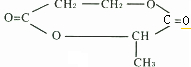





 B��CH4 C��CH2=CHCH3 D��CH3CH2C��CH E��CH3CH3
B��CH4 C��CH2=CHCH3 D��CH3CH2C��CH E��CH3CH3