��Ŀ����
��һ�̶�������ܱ������У������л�ѧ��Ӧ��CO��g��+H2O��g��?CO2��g��+H 2��g�����仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±�| T���棩 | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
A�������������䣬�����¶ȣ�CO��ת���ʱ��
B���¶����ߣ������淴Ӧ���ʶ�������ƽ���ƶ��Ĺ���������ѹǿʼ�ձ��ֲ���
C����Ӧ��ƽ��ʱ����������ƽ��Ħ������M��18��M��26
D������ijƽ��״̬ʱ��c��CO2��×c��H2��=c��CO��×c��H2O�������ʱ���¶�Ϊ830��
B���÷�ӦΪ�����������ķ�Ӧ��������������䣬�����¶ȣ�ѹǿ����
C����Ӧ�����������������䣬�ܵ����ʵ������䣬ƽ��Ħ������Ϊ��ֵ������ʼ���ʼ������ʵ����ʵ����йأ�
D����ijƽ��״̬ʱ��c��CO2��×c��H2��=c��CO��×c��H2O������˵��ƽ�ⳣ��k=1���ݴ��жϣ�
����⣺A���ɱ������ݿ�֪���¶����ߣ�ƽ�ⳣ�����������¶�ƽ��������Ӧ�ƶ���CO��ת��������A��ȷ��
B���÷�ӦΪ�����������ķ�Ӧ��������������䣬�����¶ȣ�ѹǿ����B����
C����Ӧ�����������������䣬�ܵ����ʵ������䣬ƽ��Ħ������Ϊ��ֵ����Ӧ��һ���Ǵ�����Ӧ���У�����ʼ���ʼ������ʵ����ʵ����йأ���ƽ��Ħ��������һ������18��26֮�䣬��C����
D����ijƽ��״̬ʱ��c��CO2��×c��H2��=c��CO��×c��H2O������˵��ƽ�ⳣ��k=1���ɱ������ݿ�֪�����¶�Ϊ830�棬��D��ȷ��
��ѡBC��
���������⿼�黯ѧƽ��ļ�����Ӱ�����ء���ѧƽ�ⳣ���ȣ��Ѷ��еȣ�Bѡ��Ϊ�״��㣬�ڴ��������¶ȶ�ѹǿ��Ӱ�죮

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д���14�֣�ÿ��2�֣�
I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2(g) 2NH3(g) ��H= -92.4 kJ/mol �ݴ˻ش��������⣺
��1��Ϊ����߰����IJ��ʣ�����ѡ��ķ����� _______ ������ţ���ѡ�۷֣���
�� �����¶� �� ����ѹǿ �� ʹ�ô��� �� ��ʱ�����NH3
��2�������ܱ������н��еĿ��淴Ӧ��N2(g)+3H2(g)2NH3(g)��������������£�
˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬____________________________________��
��N2������Ӧ������NH3���淴Ӧ���ʵ�1/2
���ں��������£���������ѹǿ���ֲ���
��N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
�ܵ�λʱ����ÿ����3mol H2��ͬʱ��2mol NH3����
��3mol N-N�����ѣ�ͬʱ��6mol N-H������
��3��һ�������£�NH3�ڹ̶�������ܱ������з����ֽⷴӦ����H��0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ����ǣߣߣߣ�____________��ѡ����ţ���
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ����NH3�����ʵ��� |
| y | NH3�����ʵ��� | ƽ�ⳣ��K | NH3��ת���� | ���������ʵ����ܺ� |
II����1����������Һ�У������(KIO3)���������ƿɷ������·�Ӧ��
2IO3����5SO32����2H��===I2��5SO42����H2O
���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ��
|
| 0.01mol��L��1 KIO3������Һ(������)�����/mL | 0.01mol��L��1 Na2SO3��Һ�����/mL | H2O����� /mL | ʵ�� �¶� /�� | ��Һ������ɫʱ����ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | --------- |
| ʵ��2 | 5 | 5 | 40 | 25 | ---------- |
| ʵ��3 | 5 | 5 | V2 | 0 | ----------- |
��ʵ���Ŀ����_______________________________________________________________
________________________________________________��
����V1=___________mL.
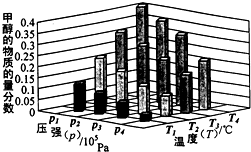
��2�����淴ӦC(s)+H2O(g) H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ����
![]() ��ʱ��t�Ĺ�ϵ����ͼ��
��ʱ��t�Ĺ�ϵ����ͼ��
��ͼ��t4��t6��ʱ����ƽ���ƶ������������� ��
��ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ���� ��

��14�֣�ÿ��2�֣� I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2 (g)
(g)  2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
��1��Ϊ����߰����IJ��ʣ�����ѡ��ķ����� _______������ţ���ѡ�۷֣���
�� �����¶� �� ����ѹǿ �� ʹ�ô��� �� ��ʱ�����NH3
��2�������ܱ������н��еĿ��淴Ӧ��N2(g)+3H2(g) 2NH3(g)��������������£�
2NH3(g)��������������£�
˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬____________________________________��
��N2������Ӧ������NH3���淴Ӧ���ʵ�1/2
���ں��������£���������ѹǿ���ֲ���
��N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
�ܵ�λʱ����ÿ����3mol H2��ͬʱ��2mol NH3����
��3mol N-N�����ѣ�ͬʱ��6mol N-H������
��3��һ�������£�NH3�ڹ̶�������ܱ������з����ֽⷴӦ����H��0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ����ǣߣߣߣ�____________��ѡ����ţ���
| ѡ�� | a | b | c | d |
| x | �¶� | �¶� | ����H2�����ʵ��� | ����NH3�����ʵ��� |
| y | NH3�����ʵ��� | ƽ�ⳣ��K | NH3��ת���� | ���������ʵ����ܺ� |
II����1����������Һ�У������(KIO3)���������ƿɷ������·�Ӧ��
2IO3����5SO32����2H��===I2��5SO42����H2O
���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ��
| | 0.01mol��L��1 KIO3������Һ(������)�����/mL | 0.01mol��L��1 Na2SO3��Һ�����/mL | H2O����� /mL | ʵ�� �¶� /�� | ��Һ������ɫʱ����ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | --------- |
| ʵ��2 | 5 | 5 | 40 | 25 | ---------- |
| ʵ��3 | 5 | 5 | V2 | 0 | ----------- |
________________________________________________��
����V1=___________mL.
��2�����淴ӦC(s)+H2O(g)
 H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ����
H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ���� ��ʱ��t�Ĺ�ϵ����ͼ��
��ʱ��t�Ĺ�ϵ����ͼ����ͼ��t4��t6��ʱ����ƽ���ƶ�������������
 ��
����ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ���� ��

��14�֣�ÿ��2�֣�
I���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2(g)
 2NH3(g) ��H= -92.4 kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H= -92.4 kJ/mol �ݴ˻ش��������⣺
��1��Ϊ����߰����IJ��ʣ�����ѡ��ķ����� _______ ������ţ���ѡ�۷֣���
�� �����¶� �� ����ѹǿ �� ʹ�ô��� �� ��ʱ�����NH3
��2�������ܱ������н��еĿ��淴Ӧ��N2(g)+3H2(g) 2NH3(g)��������������£�
2NH3(g)��������������£�
˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬____________________________________��
��N2������Ӧ������NH3���淴Ӧ���ʵ�1/2
���ں��������£���������ѹǿ���ֲ���
��N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
�ܵ�λʱ����ÿ����3mol H2��ͬʱ��2mol NH3����
��3mol N-N�����ѣ�ͬʱ��6mol N-H������
��3��һ�������£�NH3�ڹ̶�������ܱ������з����ֽⷴӦ����H��0������ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ����ǣߣߣߣ�____________��ѡ����ţ���
|
ѡ�� |
a |
b |
c |
d |
|
x |
�¶� |
�¶� |
����H2�����ʵ��� |
����NH3�����ʵ��� |
|
y |
NH3�����ʵ��� |
ƽ�ⳣ��K |
NH3��ת���� |
���������ʵ����ܺ� |
II����1����������Һ�У������(KIO3)���������ƿɷ������·�Ӧ��
2IO3����5SO32����2H��===I2��5SO42����H2O
���ɵĵ�����õ�����Һ���飬���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ��
|
|
0.01mol��L��1 KIO3������Һ(������)�����/mL |
0.01mol��L��1 Na2SO3��Һ�����/mL |
H2O����� /mL |
ʵ�� �¶� /�� |
��Һ������ɫʱ����ʱ��/s |
|
ʵ��1 |
5 |
V1 |
35 |
25 |
--------- |
|
ʵ��2 |
5 |
5 |
40 |
25 |
---------- |
|
ʵ��3 |
5 |
5 |
V2 |
0 |
----------- |
��ʵ���Ŀ����_______________________________________________________________
________________________________________________��
����V1=___________mL.
��2�����淴ӦC(s)+H2O(g)  H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ����
H2(g)+CO(g)����H>0�ﵽƽ��ı�ijһ������������ı����ʵ����������£�����Ӧ���� ��ʱ��t�Ĺ�ϵ����ͼ��
��ʱ��t�Ĺ�ϵ����ͼ��
��ͼ��t4��t6��ʱ����ƽ���ƶ������������� ��
��ͼ�б�ʾƽ��������CO�ĺ�����ߵ�һ��ʱ���� ��

 ��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�
��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�

