��Ŀ����
����ʵ����������ӷ�Ӧ����ʽ�������
- A.��NaHCO3��Һ�еμ���������ʯ��ˮ�� Ca2++2OH�D+2HCO3�D= CaCO3��+CO32�D+2H2O
- B.NaOH��Һ�м������ϡ��� H3O+ + OH�D= 2H2O
- C.����ʯ��ˮ�еμ�NaHSO4��Һ�����ԣ� Ca2+ + OH�D+H+ + SO42�D= CaSO4��+H2O
- D.Na2CO3��Һ�м�������ʵ�������� CO32�D+ CH3COOH = HCO3�D+CH3COO�D

��2013?������ģ�����и���ʵ��������������ó��Ľ�����ȷ���ǣ�������
|
��08�꽭�վ���
A����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��E�ĺ�������Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
B��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1-�嶡��ķ�Ӧ���£�
NaBr+H2SO4![]() HBr+NaHSO4 ��
HBr+NaHSO4 ��
R-OH+HBr![]() R-Br+H2O ��
R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br�D��Ũ��������ΪBr2�ȡ��й������б����£�
| �Ҵ� | ������ | ������ | 1-�嶡�� |
�ܶ�/g?cm-3 | 0��7893 | 1��4604 | 0��8098 | 1��2758 |
�е�/�� | 78��5 | 38��4 | 117��2 | 101��6 |
��ش��������⣺
��1���������1-�嶡����Ʊ�ʵ���У���������������õ����� ��������ĸ��
a��Բ����ƿ b����Ͳ c����ƿ d������©��
��2���������ˮ���� ������ڡ��������ڡ���С�ڡ�������ԭ���� ��
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã������� ����ϲ㡱�����²㡱���ֲ㡱����
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��5������ȥ������е���������Br2���������������ʺϵ��� ��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ������������� �������Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ���� ��
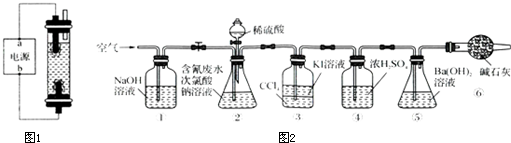
 �屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ��ͼ���й��������£������кϳɲ���ش����⣺
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ��ͼ���й��������£������кϳɲ���ش����⣺