��Ŀ����
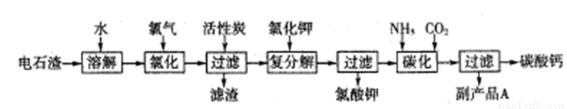
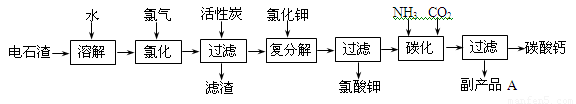
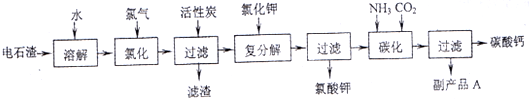
�Ե�ʯ��[��Ҫ�ɷ���Ca��OH��2����SiO2��Al2O3�Լ�������������]Ϊԭ���������������̼��Ƶ��������£�
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��______��______��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��______��
��3���������м������̿��������______��
��4����������̼����Ӧ�����ӷ���ʽΪ______��
��5������ƷA�Ļ�ѧʽΪ______��
�⣺��1����ʯ����Ҫ�ɷ���Ca��OH��2����SiO2��Al2O3����SiO2�����������Al2O3��������������߶�����ǿ��Ca��OH��2��Ӧ���ʷ�Ӧ����ʽΪCa��OH��2+SiO2 =CaSiO3+H2O��Al2O3+Ca��OH��2=Ca�� AlO2 ��2+H2O���ʴ�Ϊ��Ca��OH��2+SiO2 =CaSiO3+H2O��Al2O3+Ca��OH��2=Ca�� AlO2 ��2+H2O��
��2���������֪�������������أ�����ǰ����Ȼ���Ӧ����Ӧ����ClO3-���ʷ�Ӧ����ʽΪ6Cl2+6Ca��OH��2=Ca��ClO3��2+5CaCl2+6H2O���ʴ�Ϊ��6Cl2+6Ca��OH��2=Ca��ClO3��2+5CaCl2+6H2O��
��3������̿�����������ã��������ж�����Cl2���ʴ�Ϊ��������������ֹ�ں���ʵ���а����ݳ���Ⱦ������
��4��̼��ʱͨ��NH3��CO2 ������ˮ��������CO32-��NH4+��CO32-����Ca2+�������CaCO3�������ʴ�Ϊ��Ca2++2NH3+CO2+H2O=CaCO3��+2NH4+��
��5���ɣ�4����֪��Һ��������ΪNH4+���ٸ���ǰ�漸����֪��������Ҫ��Cl-���ʴ�Ϊ��NH4Cl��
��������1��Ca��OH��2+SiO2 =CaSiO3+H2O Al2O3+Ca��OH��2=Ca�� AlO2 ��2+H2O��
��2��6Cl2+6Ca��OH��2 Ca��ClO3��2+5CaCl2+6H2O��
Ca��ClO3��2+5CaCl2+6H2O��
��3������̿�����������ã�
��4��Ca2++2NH3+CO2+H2O=CaCO3��+2NH4+��
��5���ɣ�4����֪��Һ��������ΪNH4+���ٸ���ǰ�漸����֪��������Ҫ��Cl-��
�����������Թ�������ͼΪ���У��ۺϿ����˼��ֽ����ͷǽ�������������ʣ�ͬʱ���������ӷ�Ӧ������Ĺؼ��Ƿ���������ͼ��ע��ǰ���Ӧ���ϵ��
��2���������֪�������������أ�����ǰ����Ȼ���Ӧ����Ӧ����ClO3-���ʷ�Ӧ����ʽΪ6Cl2+6Ca��OH��2=Ca��ClO3��2+5CaCl2+6H2O���ʴ�Ϊ��6Cl2+6Ca��OH��2=Ca��ClO3��2+5CaCl2+6H2O��
��3������̿�����������ã��������ж�����Cl2���ʴ�Ϊ��������������ֹ�ں���ʵ���а����ݳ���Ⱦ������
��4��̼��ʱͨ��NH3��CO2 ������ˮ��������CO32-��NH4+��CO32-����Ca2+�������CaCO3�������ʴ�Ϊ��Ca2++2NH3+CO2+H2O=CaCO3��+2NH4+��
��5���ɣ�4����֪��Һ��������ΪNH4+���ٸ���ǰ�漸����֪��������Ҫ��Cl-���ʴ�Ϊ��NH4Cl��
��������1��Ca��OH��2+SiO2 =CaSiO3+H2O Al2O3+Ca��OH��2=Ca�� AlO2 ��2+H2O��
��2��6Cl2+6Ca��OH��2
 Ca��ClO3��2+5CaCl2+6H2O��
Ca��ClO3��2+5CaCl2+6H2O����3������̿�����������ã�
��4��Ca2++2NH3+CO2+H2O=CaCO3��+2NH4+��
��5���ɣ�4����֪��Һ��������ΪNH4+���ٸ���ǰ�漸����֪��������Ҫ��Cl-��
�����������Թ�������ͼΪ���У��ۺϿ����˼��ֽ����ͷǽ�������������ʣ�ͬʱ���������ӷ�Ӧ������Ĺؼ��Ƿ���������ͼ��ע��ǰ���Ӧ���ϵ��

��ϰ��ϵ�д�
�����Ŀ