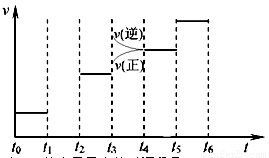
��Ŀ����
(9��)��һ���¶��£���һ���ݻ�����������м���3 mol SO2��2 mol O2�����������������Ӧ: 2SO2��g����O2��g��=2SO3��g�� ��H����196.6 kJ/mol��ƽ��ʱ����������ѹǿΪ��ʼʱ��90%��
(1)����3mol SO2��3mol O2������Ӧ,�ﵽƽ��ʱ,SO2��ת����Ϊ_____��
(2)�����¶Ȳ��䣬����ͬ�������У�����ʼ���ʵ����ʵ�����Ϊa mol SO2��b mol O2��c mol SO3(g)(c>0)����ʹƽ��ʱSO3���������Ϊ2/9��O2���������Ϊ3/9����
�ٴﵽƽ��ʱ��(1)��(2)�ų�������________(�����)��
A����ȡ� B��ǰ��С�ں��� C��ǰ�ߴ��ں��� D����ȷ��
��a.b.c��������Ĺ�ϵ��(һ����a.c��ʾ����һ����b.c��ʾ):__ _��
��9�֣���(1)33.3% (2) ��C����a��c��3��b��c/2��2
��������2SO2��g����O2��g��=2SO3��g��
��n�� 3 2 0
�Sn: 1 0. 5 1
ƽn: 2.5 1.5 1��ƽ��ʱ��������������ʵ���֮��Ϊ��ʼʱ��90%��
��1��SO2��ת����Ϊ1/3
��2������ƽ��ʱSO3���������Ϊ2/9��O2���������Ϊ3/9��֪������Ŀ����Ϊ��Чƽ�⣬������c>0���ʴﵽƽ��ʱ��(1)��(2)�ų�������ǰ�ߴ�
�ڸ��ݵ�������µ�Чƽ��ij���������֪��a��c��3��b��c/2��2

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�(9��)��һ���¶��£���һ���ݻ�����������м���3 mol SO2��2 mol O2�����������������Ӧ:2SO2��g����O2��g��=2SO3��g����H����196.6 kJ/mol��ƽ��ʱ����������ѹǿΪ��ʼʱ��90%��
(1)����3mol SO2��3mol O2������Ӧ,�ﵽƽ��ʱ,SO2��ת����Ϊ_____��
(2)�����¶Ȳ��䣬����ͬ�������У�����ʼ���ʵ����ʵ�����Ϊa mol SO2��b mol O2��c mol SO3(g)(c>0)����ʹƽ��ʱSO3���������Ϊ2/9��O2���������Ϊ3/9����
�ٴﵽƽ��ʱ��(1)��(2)�ų�������________(�����)��
| A����ȡ� | B��ǰ��С�ں��� | C��ǰ�ߴ��ں��� | D����ȷ�� |
 ��
�� ��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ
��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ
 ����֪N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ
����֪N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ 2NH3(g)����H<0 ��
2NH3(g)����H<0 ��