��Ŀ����
 [��ѧ-��ѧ�뼼��]
[��ѧ-��ѧ�뼼��]Ԫ��������������������Ӧ�ù㷺�Ľ���Ԫ�أ�
��1���ӿ�ʯ��ȡ����������ʯ��NaOH��Һ���·�Ӧ��Ȼ�����������壬�پ������������յõ���������������������ʱ�ķ�Ӧ����ʽΪ��
��2���������ǹ�ҵ���ұ��������Ҫԭ�ϣ������м������ʯ��Na3AlF6������������
�������ķ�Ӧʽ
���ڵ��ع��������У���Ҫ���ϲ����������ϣ�ԭ����
��3�������Ͻ��ܣ���Һ����Ԫ������������[��Al��OH��3��ʾ]��ʽ���ڵ�pH��Χ��3.8��10������A��B���־�����Ԫ���γɵ�ij��������Һ����pH�ֱ�Ϊ1��13������Һ��������ʱ��Ӧ�����ӷ���ʽΪ
��4��һ������Ч��ˮ��PAFC--�ۺ��Ȼ�����[A1Fe��OH��nCl6-n]m���㷺�����ճ�������ˮ��ҵ��ˮ�Ĵ������й�PAFC��˵����ȷ����
A��PAFC����Ԫ����+2��
B��PAFC���ھ�ˮʱ��������ͬ�����Ȼ������Ȼ�����ˮ��pH�ı�С
C��PAFC�ɿ���һ���������Ȼ������Ȼ���ˮ����м����
D��PAFC��ǿ���Ժ�ǿ������Һ�ж����ȶ����ڣ�
��������1����ʯ��NaOH��Һ���·�Ӧ��Ȼ�����������壬�پ������������յõ���������˵����������ΪAl��OH��3��
��2���������۵���������ڵ�⣬�����м������ʯ��Na3AlF6���������������۵㣬�������ڵ�⣻
�ٵ����������������õ�������������
�ڵ����������������������ʧ���ӷ���������Ӧ���������������͵缫ʯī��Ӧ���ɶ�����̼��ĵ缫��
��3������������ˮ�������ԣ����ǻ������������ˮ�� �Լ��ԣ���Ϻ�ˮ����ٽ�������������������
��4��A�����ݻ�����Ԫ�ػ��ϼ۴�����Ϊ0�������㣻
B�������ھ���ˮʱ��ˮ��pH�ĸı䣬����ͬ����AlCl3��FeCl3����
C�����ּ�ʽ�οɿ���FeCl3��AlCl3ˮ����м���
D������ǿ���Ժ�ǿ������Һ�ж������ȶ����ڣ�
��2���������۵���������ڵ�⣬�����м������ʯ��Na3AlF6���������������۵㣬�������ڵ�⣻
�ٵ����������������õ�������������
�ڵ����������������������ʧ���ӷ���������Ӧ���������������͵缫ʯī��Ӧ���ɶ�����̼��ĵ缫��
��3������������ˮ�������ԣ����ǻ������������ˮ�� �Լ��ԣ���Ϻ�ˮ����ٽ�������������������
��4��A�����ݻ�����Ԫ�ػ��ϼ۴�����Ϊ0�������㣻
B�������ھ���ˮʱ��ˮ��pH�ĸı䣬����ͬ����AlCl3��FeCl3����
C�����ּ�ʽ�οɿ���FeCl3��AlCl3ˮ����м���
D������ǿ���Ժ�ǿ������Һ�ж������ȶ����ڣ�
����⣺��1����ʯ��NaOH��Һ���·�Ӧ��Ȼ�����������壬�پ������������յõ���������˵����������ΪAl��OH��3����ʯ�������������������������������ǻ��������Ƹ��·ֽ����������������������ƣ���������ķ�ӦΪNaAl��OH��4=Al��OH��3��+NaOH��
�ʴ�Ϊ��NaAl��OH��4=Al��OH��3��+NaOH��
��2���������۵���������ڵ�⣬�����м������ʯ��Na3AlF6���������������۵㣬�������ڵ�⣬�����������۵㣬ʹ����1000�����Ҽ����ۻ���
�ʴ�Ϊ�����������ۼ���
�ٵ����������������õ��������������缫��ӦΪ��Al3++3e-=Al���ʴ�Ϊ��Al3++3e-=Al��
�ڵ����������������������ʧ���ӷ���������Ӧ���������������͵缫ʯī��Ӧ���ɶ�����̼���缫ʯī���ϱ����ģ�������Ҫ���ϲ���ʯī�缫��
�ʴ�Ϊ�������������O2���ڸ��������£�ʯī���������ϵ�����ΪCO2��
��3��A��B���־�����Ԫ���γɵ�ij��������Һ����pH�ֱ�Ϊ1��13��˵����Һ����������ΪAl3+ˮ�⣬��Һ�ʼ���ΪNaAl��OH��4��Һˮ���Լ��ԣ���Ϻ�ˮ����ٽ���Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3++3[Al��OH��4]-=4Al��OH��3�����ʴ�Ϊ��Al3++3[Al��OH��4]-=4Al��OH��3����
��4��A���������ܼ�̬��6-n+n=6����ֻ��+3�ۣ�����Fe����6-3=3�������mֻ�DZ�ʾ����һ�����Ӿۺ϶ȣ����̬�أ���A����
B��PAFC���ھ�ˮʱ�����������ӵĴ��ڶ�ˮ�ĵ����ڴ��������ã�������ͬ�����Ȼ������Ȼ�����ˮ��pH�ı�С����B��ȷ��
C��PAFC�Ǽ�ʽ���οɿ���һ���������Ȼ������Ȼ���ˮ����м�����C��ȷ��
D��PAFC�Ǽ�ʽ���Σ���ǿ���Ժ�ǿ������Һ�ж������ȶ����ڣ���D����
�ʴ�Ϊ��BC��
�ʴ�Ϊ��NaAl��OH��4=Al��OH��3��+NaOH��
��2���������۵���������ڵ�⣬�����м������ʯ��Na3AlF6���������������۵㣬�������ڵ�⣬�����������۵㣬ʹ����1000�����Ҽ����ۻ���
�ʴ�Ϊ�����������ۼ���
�ٵ����������������õ��������������缫��ӦΪ��Al3++3e-=Al���ʴ�Ϊ��Al3++3e-=Al��
�ڵ����������������������ʧ���ӷ���������Ӧ���������������͵缫ʯī��Ӧ���ɶ�����̼���缫ʯī���ϱ����ģ�������Ҫ���ϲ���ʯī�缫��
�ʴ�Ϊ�������������O2���ڸ��������£�ʯī���������ϵ�����ΪCO2��
��3��A��B���־�����Ԫ���γɵ�ij��������Һ����pH�ֱ�Ϊ1��13��˵����Һ����������ΪAl3+ˮ�⣬��Һ�ʼ���ΪNaAl��OH��4��Һˮ���Լ��ԣ���Ϻ�ˮ����ٽ���Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3++3[Al��OH��4]-=4Al��OH��3�����ʴ�Ϊ��Al3++3[Al��OH��4]-=4Al��OH��3����
��4��A���������ܼ�̬��6-n+n=6����ֻ��+3�ۣ�����Fe����6-3=3�������mֻ�DZ�ʾ����һ�����Ӿۺ϶ȣ����̬�أ���A����
B��PAFC���ھ�ˮʱ�����������ӵĴ��ڶ�ˮ�ĵ����ڴ��������ã�������ͬ�����Ȼ������Ȼ�����ˮ��pH�ı�С����B��ȷ��
C��PAFC�Ǽ�ʽ���οɿ���һ���������Ȼ������Ȼ���ˮ����м�����C��ȷ��
D��PAFC�Ǽ�ʽ���Σ���ǿ���Ժ�ǿ������Һ�ж������ȶ����ڣ���D����
�ʴ�Ϊ��BC��
���������⿼���˵��ԭ����Ӧ�÷����������̵ķ�Ӧ�жϣ��缫��д��������������ɽṹ����������ʵ��жϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����ѧ-��ѧ�뼼����ijǿ���Թ�ҵ��ˮ�к���Fe2+��Fe3+��Cu2+�����ʵ�鷽���ó�������ȥ�����ӣ��õ��ϴ���Cu2+��Һ���й��������������pH���±���
��1���ӱ������ݷ�����Ϊʲô����ֱ�Ӽ�NaOH����ҺpH����9.7����ȥFe3+��Fe2+ ��
��2��ʵ��Ӧ���������ȼ�һ�����Ĵ������ƣ�Ȼ���ٵ�����ҺpH���������Ƶ������� ��
��3��pHӦ���ڵ�ʲô��Χ ��Ϊʲô ��
��4������pH���˵��Լ���
A����������B��̼��þ��C������ͭ��D��ϡ����
���� ��
| �������� | pH | |
| ��ʼ���� | ������ȫ | |
| Fe2+ | 6.3 | 9.7 |
| Cu2+ | 4.7 | 6.7 |
| Fe3+ | 1.9 | 3.2 |
��2��ʵ��Ӧ���������ȼ�һ�����Ĵ������ƣ�Ȼ���ٵ�����ҺpH���������Ƶ�������
��3��pHӦ���ڵ�ʲô��Χ
��4������pH���˵��Լ���
A����������B��̼��þ��C������ͭ��D��ϡ����
����
����ѧ--��ѧ�뼼����
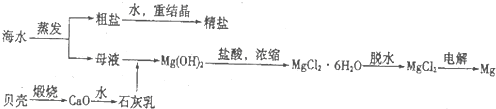
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
��1�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ1�������� ���������ӽ���Ĥֻ����������������ͨ����
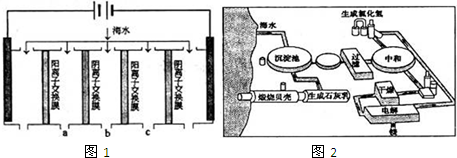
�������ĵ缫��ӦʽΪ ��
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ ��
�۵�ˮ�ij���Ϊa��b��c�е� ���ڣ�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ��������� ��
���������������У�ѭ��ʹ�õ������� ��
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵��� ���ͬ�⡱��ͬ�⡱���������� ��
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO 42- | HCO 3+ | ����/mg?L-1 | 9360 | 83 | 200 | 1100 | 16000 | 1200 | 118 |

�������ĵ缫��ӦʽΪ
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ
�۵�ˮ�ij���Ϊa��b��c�е�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ���������
���������������У�ѭ��ʹ�õ�������
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵���