��Ŀ����
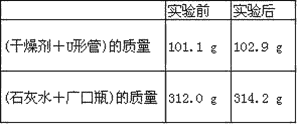
����Ŀ����ѧ�������һ��ʹ�ù������ʵ�ȼ�ϵ�أ���Ч�ʸ��ߣ������ں��캽�ա���ͼ1��ʾװ���У���ϡ���������������Ե缫���ڵ缫�Ϸֱ�ͨ�����Ϳ��������й��������Dz�����Y2O3��ZrO2���壬���ڸ������ܴ���O2��(O2��4e��===2O2��)��

(1)c�缫������Ϊ__________��������������������������d�缫�ϵĵ缫��ӦʽΪ_________
(2)��ͼ2��ʾ�ö��Ե缫���100mL0.5mol��L��1����ͭ��Һ��a�缫�ϵĵ缫��ӦʽΪ_______��b�缫�ϵĵ缫��ӦʽΪ ____________________,��a�缫����56mL(��״��)���壬���·��ת�Ƶĵ���Ϊ__________mol��������Һ��pH��________(��������Һ����仯)����Ҫʹ�������Һ�ָ������ǰ��״̬���ɼ���________(����ĸ)��
a��CuO b��Cu(OH)2 c��Cu d��Cu2(OH)2CO3
���𰸡����� CH4��4O2����8e��===CO2��2H2O 4OH����4e��===2H2O��O2������2H2O��4e��===O2��+4H���� Cu2++2e��===Cu 1��10��2 1 a
��������
ͼ1Ϊԭ��أ����ݵ�������d�缫Ϊ����������ʧ���ӣ��������ӷ�Ӧ���ɶ�����̼��ˮ��c�������õ������������ӣ�ͼ2Ϊ���أ�aΪ�������缫Ϊ���Ե缫����Һ�е�ˮʧ�������������������ӣ�b��Ϊ������ͭ���ӵõ��ӣ�����ͭ��
(1)ͼ1Ϊԭ��أ����ݵ���������c�缫Ϊ������d��Ϊ����������ʧ���ӣ��������ӷ�Ӧ���ɶ�����̼��ˮ���缫��ӦʽΪCH4��4O2����8e��==CO2��2H2O��
(2)��ͼ2��ʾ�ö��Ե缫��⣬a��Ϊ��������Һ�е�ˮʧ�������������������ӣ��缫��ӦʽΪ4OH����4e��===2H2O��O2������2H2O��4e��===O2��+4H������b��ͭ���ӵõ�������ͭ���缫��ӦʽΪCu2++2e��===Cu������56mL����µ�����ʱ����0.0025mol��ת��0.01mol���ӣ�����0.01mol�����ӣ�c(H+)=![]() =0.1mol/L����pH=1��a������0.0025mol������b������0.005molCu����Ҫ�ָ������ǰ����Һ�������0.005molCuO���ɣ���Ϊa��
=0.1mol/L����pH=1��a������0.0025mol������b������0.005molCu����Ҫ�ָ������ǰ����Һ�������0.005molCuO���ɣ���Ϊa��