��Ŀ����
���и���������ָ����Һ���ܴ����������
[ ]
�ٳ����£�c(H+)/c(OH-)��1��10-12����Һ��K+��AlO2-��CO32-��Na+
�ڼ��뱽������ɫ����Һ��K+��NH4+��Cl-��I-
��������Һ��Fe3+��Al3+��NO3-��SO42-
��ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl-
����ɫ��Һ�У�K+��AlO2-��NO3-��HCO3-
A���ڢۡ���
B���٢ۡ���
C���١� ��
D���٢ܢ�
�ڼ��뱽������ɫ����Һ��K+��NH4+��Cl-��I-
��������Һ��Fe3+��Al3+��NO3-��SO42-
��ʹpH��ֽ��������Һ�У�NH4+��Na+��SO42-��Cl-
����ɫ��Һ�У�K+��AlO2-��NO3-��HCO3-
A���ڢۡ���
B���٢ۡ���
C���١� ��
D���٢ܢ�
C

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��Na+��HSO
��Na+��HSO ��PO
��PO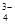 ��MnO
��MnO
 ��NO
��NO ��SO
��SO
 ��CO
��CO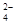 ��Cl
��Cl =0.1 mol/L����Һ�У�Na+��K+��SO
=0.1 mol/L����Һ�У�Na+��K+��SO