��Ŀ����
(8��)(1)2003��3���ձ��������Ͽ�ѧ����ʵ����һ���о�С�鷢���������ᾧˮ�ľ�����5K�³��ֳ����ԡ��ݱ������þ���Ļ�ѧʽΪ Na0.35CoO2 • 1.3H2O���Լ��㣺�þ�������ԭ������ԭ�ӵ����ʵ���֮����___��_____�� 1mol�þ����к��е���ԭ����Ŀ��____��_______��
(2)���ơ��⡢����������Ԫ�أ������е�һ�ֻ���Ԫ�ؿ�����ɶ������ʣ�д����������Ҫ��Ļ�ѧ����ʽ��
������������ͼӦ������������������������������������������
�ڼ�����������ᷴӦ�������������� ����

����������

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�(8��)�й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%~50%��
��1����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���
A�����ˮ���⣺2H2O 2H2����O2��
2H2����O2��
B������ʹˮ�ֽ����⣺2H2O 2H2����O2��
2H2����O2��
C��̫������ֽ�ˮ���⣺2H2O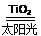 2H2����O2��
2H2����O2��
D����Ȼ�����⣺CH4��H2O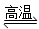 CO��3H2
CO��3H2
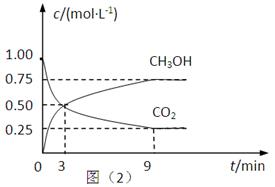
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0kJ��mol��1�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
�ٴ�3 min��9 min��v(H2)=________mol��L��1��min��1��
����˵��������Ӧ�ﵽƽ��״̬����____________�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3mol H2��ͬʱ����1mol H2O
D��CO2����������ڻ�������б��ֲ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ����
CH3OH(g)��ƽ�ⳣ����
|
�¶�/�� |
0 |
100 |
200 |
300 |
400 |
|
ƽ�ⳣ�� |
667 |
13 |
1.9��10-2 |
2.4��10-4 |
1��10-5 |
����˵����ȷ����_____��
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S��0
C����T��ʱ��1L�ܱ������У�Ͷ��0.1mol CO��0.2 mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ(5Mpa)��250�棬����Ϊ�������£�ԭ����ת�������
(8��)�±���ʾ�ϳɰ���Ӧ��N2+3H2  2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
2NH3���ڲ�ͬ�����´ﵽƽ��ʱ������а��ĺ���[��ʼʱv��N2����v��H2��==1��3]��
|
�¶ȣ��棩 |
0.1 |
10 |
30 |
60 |
100 |
|
200 |
0.153 |
0.815 |
0.899 |
0.954 |
0.988 |
|
300 |
0.022 |
0.520 |
0.710 |
0.842 |
0.926 |
|
400 |
0.004 |
0.251 |
0.470 |
0.652 |
0.798 |
�����ϱ�����,�ش��������⣺
��1��200�桢100MPaʱ��ƽ�������а��ĺ����Ѵ�0.988�������������ѹǿ
����ܡ����ܡ���ʹƽ�������а��ĺ�������1�������ǣ�
��
��2����ʹƽ�������а��ĺ���������ɲ�ȡ�Ĵ�ʩ�У� ��
��3�� ��ʹƽ�������а��ĺ���Ϊ0.710����ѡ��ķ�Ӧ����ӦΪ��
��ʹƽ�������а��ĺ���Ϊ0.710����ѡ��ķ�Ӧ����ӦΪ��
 2NH3�ġ�H
2NH3�ġ�H ѹǿ��MPa��
ѹǿ��MPa�� ���ĺ���
�����