��Ŀ����
����Ŀ����.���з�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H<0����850��ʱ��K��1��
CO2(g)��H2(g) ��H<0����850��ʱ��K��1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK__________1(����ڡ���С�ڡ����ڡ�)��
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��x mol H2����
�ٵ�x��5.0ʱ������ƽ����_____________(�����Ӧ�����淴Ӧ��)�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������_______________��
��.��һ�̶��ݻ����ܱ�������,����һ���¶�,��һ�������·������·�Ӧ:2A(g)+B(g)![]() 3C(g),��֪����1 mol A��2 mol B�Ҵﵽƽ���,������a mol C��
3C(g),��֪����1 mol A��2 mol B�Ҵﵽƽ���,������a mol C��
��3���ﵽƽ��ʱ,C�ڷ�Ӧ��������е����������___(�ú�a�Ĵ���ʽ��ʾ)��
��4������ͬ��ʵ��������,����ͬһ�����и�Ϊ����2 mol A��4 mol B,�ﵽƽ���,C�����ʵ���Ϊ___mol(�ú�a�Ĵ���ʽ��ʾ)����ʱC�ڷ�Ӧ��������е����������ԭƽ�����___(��������������С������������)��
��5������ͬʵ��������,����ͬһ�����и�Ϊ����2 mol A��5 mol B,��Ҫ��ƽ���C�ڷ�Ӧ��������е������������ԭƽ����ͬ,��Ӧ����___mol C��
���𰸡�С�� �淴Ӧ x��3.0 ![]() 2a ���� 1
2a ���� 1
��������
��1��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H<0������Ӧ���ȣ��������¶ȣ�ƽ�������ƶ��������¶ȵ�950��ʱ�� KС��1��
CO2(g)��H2(g) ��H<0������Ӧ���ȣ��������¶ȣ�ƽ�������ƶ��������¶ȵ�950��ʱ�� KС��1��
��2����850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��0.5 mol H2��Q=![]() >K=1������ƽ�����淴Ӧ�����ƶ���
>K=1������ƽ�����淴Ӧ�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���������Q<K��Q=![]() <K=1��x��3.0��
<K=1��x��3.0��
��3����Ӧǰ���������ʵ������䣬���Է�Ӧ������������ʵ���Ϊ3mol���ﵽƽ��ʱ��C�ڷ�Ӧ��������е����������![]() ��
��
��4����Ӧǰ���������ʵ������䣬����2 mol A��4 mol B�����1 mol A��2 mol BΪ��Чƽ�⣻�ﵽƽ���,C�����ʵ���Ϊ2amol����ʱC�ڷ�Ӧ��������е����������ԭƽ����Ȳ��䣻
��5����Ӧǰ���������ʵ������䣬Ͷ�ϱ���ͬΪ��Чƽ�⣬��Ӧ����xmol C����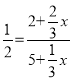 ����x=1mol��
����x=1mol��

����Ŀ��ij���жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5(ֱ��С�ڵ���2.5��m������������)������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
(1)PM2.5��ɢ�ڿ������γɵķ�ɢϵ________(����ڡ������ڡ�)���塣
(2)��PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K�� | Na�� | NH4+ | SO42- | NO3- | Cl�� |
Ũ��/ mol��L��1 | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
���ݱ��������жϴ�������Ϊ________(��ᡱ�)�ԣ���ʾ����������Ե�c(H��)��c(OH��)��________mol��L��1��
(3)Ϊ����SO2���ŷţ�����ijЩ��Һϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����__________________________(����ĸ)��
a.Ca(OH)2����b.Na2CO3����c.CaCl2����d.NaHSO3
(4)����β����NOx��CO�����ɼ�ת����
�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��д������������NO�Ļ�ѧ����ʽ��_____________________________��
������ȼ�Ͳ���ȫȼ��ʱ����CO��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NO����Ⱦ���仯ѧ��Ӧ����ʽΪ__________________________��