��Ŀ����
����Ŀ������ѧ�����ʽṹ�����ʡ�M��R��X��YΪԭ��������������Ķ���������Ԫ�أ�Z��һ�ֹ���Ԫ�ء�M��̬ԭ��L����p�����������s���ӵ�2����R��ͬ����Ԫ��������õĽ���Ԫ�أ�X��M�γɵ�һ�ֻ������������������Ҫ������Ⱦ�Z�Ļ�̬ԭ��4s��3d������������ش��������⣺
��1��R��̬ԭ�ӵĵ����Ų�ʽ�� ��X��Y�е縺�Խϴ���� ����Ԫ�ط��ţ���
��2��X���⻯��ķе��������������Ƶ�M���⻯���ԭ����________________��
��3��X��M�γɵ�XM3���ӵĿռ乹����__________��
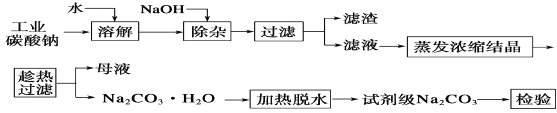
��4��M��R���γɵ�һ�����ӻ�����R2M����ľ�������ͼ��ʾ����ͼ�д��������������_________�������ӷ��ţ���

��5����ϡ�����У�Z����ۺ�����ļ��Σ���ɫ������M��һ���⻯�Z����ԭΪ+3�ۣ��÷�Ӧ�Ļ�ѧ����ʽ��_________��
��6�����������ⳤΪa cm ,��R2M������ܶ�Ϊ=___________g��cm�C3��
���𰸡���1��[Ne]3s1��1s22s22p63s1��Cl
��2��H2O����֮�����γ��������H2S����
��3��ƽ����������4��O2-
��5��3H2O2+K2Cr2O7+4H2SO4=Cr2(SO4)3+3O2��+7H2O+K2SO4
��5��248/a3��6.02��1023
��������
�����������������֪M��R��X��YΪԭ��������������Ķ���������Ԫ�أ�Z��һ�ֹ���Ԫ�ء�M��̬ԭ��L��p�������s�����������2������M��OԪ�أ�R��ͬ����Ԫ��������õĽ���Ԫ�أ���R��NaԪ�أ�X��M�γɵ�һ�ֻ��������γ��������Ҫ������Ⱦ���X��SԪ�أ�YΪClԪ�أ�Z�Ļ�̬ԭ��4s��3d�������������۵����Ų�ʽΪ3d54s1��Z��CrԪ�ء�
��1��R��NaԪ�أ�ԭ������Ϊ11�����ݹ���ԭ��ȷ�����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s1��[Ne]3s1��S��Cl�Ƚϵ縺�Խϴ����ClԪ�ء�
��2��H2S�ķе����H2O����Ҫԭ����ˮ���Ӽ��γ������ʹˮ�ķе����ߡ�
��3��SO3����ԭ�ӵļ۲���Ӷ�Ϊ3+��6-3��2��/2=3��û�йµ��Ӷԣ��÷��ӵĿռ乹��Ϊƽ�������Ρ�
��4�����ݾ����ṹ�����������ĸ���Ϊ![]() ��4������ĸ���Ϊ8���������������ӻ�����Ļ�ѧʽΪNa2O�������������Na+��������O2-��
��4������ĸ���Ϊ8���������������ӻ�����Ļ�ѧʽΪNa2O�������������Na+��������O2-��
��5����������֪�ظ���ر���ԭΪCr3+����������ⱻ�����������������û��ϼ���������ƽ����Ӧ�Ļ�ѧ����ʽΪ��K2Cr2O7+3H2O2+ 4H2SO4��K2SO4+Cr2(SO4)3+3O2��+7H2O��
��6�����������ⳤΪa cm����R2M������ܶ�Ϊ= ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��

��1��д���������������ƣ�
a. b. c. e.
��2��������װ��I�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ������� ������ˮ�� ����f��g����ͨ�롣
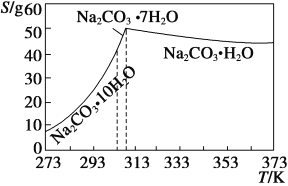
��3����������0.1mol/LNaOH��Һ450mL��װ��II��ijͬѧת����Һ��ʾ��ͼ��
��ͼ�еĴ����� ��
�����ݼ����֪����������ƽ�������NaOH������Ϊ g��
������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ |
B��������������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽⲢ��ȴ������ |
C�����ܽ������������Һ�ز�����ע��500mL������ƿ�� |
D��������ƿ�ǽ�����ҡ�� |
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2~3cm��
��4�����ʵ���Ũ��������������ƫ�ߡ�ƫ�͡���Ӱ�죩
������ֽ������������ ��
������ʱ�����۾����ӿ̶��ߣ��������Ƶ���ҺŨ�Ƚ� ��
��δ��ȴ�����¾�ע������ƿ���� ��
����õ���Һת��ɾ����Լ�ƿʱ����������������Һ ��
����Ŀ��ʵ����Ҫ0��80 mol��L-1NaOH��Һ475 mL��0��40 mol��L-1������Һ500 mL��������������Һ����������ش��������⣺
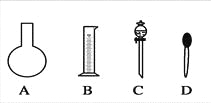
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����________������ţ�������������Һ�����õ��IJ���������________�����������ƣ���
��2�����в����У�����������ƿʵ�ֵ���______________������ţ���
A������һ�����ȷŨ�ȵı���Һ |
B����ȡһ�������Һ�� |
C����������ƿ������µ����������Һ�� |
D��ȷϡ��ijһŨ�ȵ���Һ |
E��������Һ
F�����������ܽ��������
��3�����ݼ�����������ƽ��ȡNaOH������Ϊ___________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��_________��������������������������С��������ͬ��0��8 mol��L-1��������ʱ������������ˮ����������ƿ�⣬��������ҺŨ��__________0.8 mol��L-1��
��4�����ݼ����֪��������������Ϊ98 %���ܶ�Ϊ1��84 mol��L-1��Ũ��������Ϊ__________mL������������һλС���������ʵ������10 mL��15 mL��20 mL��50 mL��Ͳ��ѡ��___________mL��Ͳ��á�