��Ŀ����
����Ŀ�����������ҹ�ҩѧ����������1971�귢�ֵ�һ�ֺ��й������ŵı�������������ɫ��״���壬������ˮ���������Ҵ���ʯ���ѡ������л��ܼ����۵�Ϊ156�棬�е�389.9�棬���ȶ��Բһ�ֳ�����ȡ�����ص���Ҫ����������ͼ��

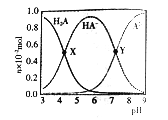
��֪��ʯ���ѵķе�Ϊ30��80�棻��������95%�Ҵ��е��ܽ�����¶ȵ����߶�����
��1��������ȡ��ԭ������ǿ��ij����������£�ʹ����ϸ���黯�����顢��ɢ�����ŵ���______�ȣ���һ�㼴�ɣ���
��2������1��������______��
����2Ӧѡ�ĺ���װ����______�����ţ��������ش�Ʒ��______���������ƣ��С�
����3�IJ���������Ũ����______�����ˡ�ϴ�ӡ����

��3������3�����¶ȹ��ߣ��ή�������صIJ��ʣ���ԭ�������______��
��4��������ֻ��C��H��O����Ԫ�ء�ijѧ��������ͼװ�ã����ȼ��г�װ��ʡ�ԣ��ⶨ�����ʽCxHyOz��

�ٸ�ʵ��װ�ÿ��ܻ��������ɲⶨ������ƫ�ͣ��Ľ�������______��
��ȡ2.82g��������Ʒ���øĽ����װ�ý���ʵ�顣ʵ�����װ��D����1.98g��װ��E����6.60g����x��y��z=______������������ȣ���Ҫȷ�������صķ���ʽ�����������������______��
���𰸡���ȡ���ʼӿ����߽�ȡ�� ���� �� ������ƿ ��ȴ�ᾧ ���������ȶ��Բ�� ��Eװ�ú�������һ��Eװ�ã��������ɣ� 15��22��5 �����ص���Է��������������ص�Ħ������
��������
(1)�黯�����顢��ɢ���ӷ�Ӧ�Ӵ�������ӿ췴Ӧ���ʣ�������ǶȽ��з�����
(2)�������̣�����1�õ���������ȡҺ��������1Ϊ���ˣ�����2�Ƿ���ʯ���Ѻʹ�Ʒ������2Ϊ�������������������95%�Ҵ��е��ܽ�����¶ȵ����߶�������з���������3��
(3) ������������95%�Ҵ��е��ܽ�����¶ȵ����߶�������з����Լ����������ȶ��Բ���з�����
(4)������ȼ�ղ���CO2��H2O����Ũ���ὫH2O���գ���ǰ���������ΪH2O���������ü�ʯ������CO2��ǰ��������ΪCO2������������ԭ���غ㣬�ó���������C��H�����ʵ������������������ص��������ó�Oԭ�ӵ����������ʵ������ݴ˷�����
(1)ʹ�������黯�����顢��ɢ������Ӵ������ʹ��ȡ���ʼӿ����߽�ȡ�ʣ�
(2)�������̣�����1�õ���������ȡҺ���ò��������ǹ��ˣ�����2�õ�ʯ���Ѻʹ�Ʒ���ò�������������������������������¶ȼƵ�ˮ����Ӧ��������ƿ֧�ܿڴ�����ȴˮӦ���½��ϳ�����Ϊ�������ʯ���ѣ�����ʯ���ѵķе���30��80�棬Ӧ����ˮԡ���ȣ��ʢ���ȷ�������صķе�Ϊ389.9�棬��������ش�ƷӦ��������ƿ�У������ص��۵���156�棬������Ϊ���壬��������95%���Ҵ��е��ܽ�����¶ȵ����߶�������˲���3�IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
(3) �������⣺��������95%���Ҵ��е��ܽ�����¶ȵ����߶��������ȶ��Բ�¶ȹ��ߣ���������ص��ܽ��������������ת�����������ʣ���������صIJ��ʽ��ͣ�
(4) ������ȼ�ղ���CO2��H2O����Ũ���ὫH2O���գ���ǰ���������ΪH2O���������ü�ʯ������CO2��ǰ��������ΪCO2������������ԭ���غ㣬�ó���������C��H�����ʵ������������������ص��������ó�Oԭ�ӵ����������ʵ�����
��װ��E�����ֱ����ͬ��������CO2��H2O(g)����װ��E������CO2��������ʹ��ԭ�Ӻ���ƫ�ͣ��Ľ���������Eװ�ú�������һ��Eװ�õȣ�
��Dװ������1.98g��Dװ�����յ�ˮ����������m(H2O)=1.98g��������ԭ���غ㣬�л�����n(H)=![]() =0.22mol��װ��E���ص�����ΪCO2�������л�����n(C)=
=0.22mol��װ��E���ص�����ΪCO2�������л�����n(C)= ![]() =0.15mol���л�����n(O)=
=0.15mol���л�����n(O)=![]() =0.05mol�������C��H��O=0.15��0.22��0.05=15��22��5��Ҫȷ�������صķ���ʽ���������������Ϊ�����ص�Ħ����������Է���������
=0.05mol�������C��H��O=0.15��0.22��0.05=15��22��5��Ҫȷ�������صķ���ʽ���������������Ϊ�����ص�Ħ����������Է���������