��Ŀ����
������������Ҫ�Ļ���ԭ��
���е�Լ77�森��ˮ�ⷴӦ����ʽΪ��CH3COOC2H5��H2O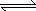 CH3COOH��C2H5OH��
CH3COOH��C2H5OH��
ʵ�鲽�裺
���Թ��м���8 mL��NaOH��Һ���ټ���2 mL������������ֱ���������������ĸ߶ȣ��ٰ��Թܷ���70���ˮԡ�У�ÿ��1 min����ȡ���������ã�������������¼ʣ������ĸ߶ȣ���Ѹ�ٷŻ�ˮԡ�м������ȣ���˷������У��ı�NaOH��ҺŨ�ȣ��ظ�ʵ�飮
���ݼ�¼����������ˮ���ʣ��ĸ߶�/min
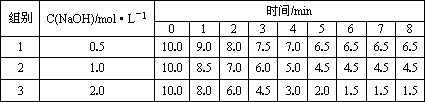
�ش��������⣺
(1)��������Ա�ʵ��ʱ��ÿ��ʵ�鶼������Ʋ����������NaOH��Һ���������������������________��
(2)�����������ݣ��õ���������ˮ�����ʵĽ����ǣ�
��NaOH��ҺŨ��Խ��ˮ������Խ________��
����������ˮ�������ȿ������һ��ʱ���ֹͣˮ�⣮
(3)���ۢڵ����۽�����________��
(4)��ʹ����������ȫˮ�⣬�ɲ�ȡ�Ĵ�ʩ��________��
(5)��ͬѧ��Ϊ�б�Ҫ������ˮ����NaOH��Һ�ظ����飬�����ݽ�����������Ҫԭ���ǣ�________��
������
|
����(1)��Ӧ�¶�(2��) ����(2)�ٿ�(2��) ����(3)NaOH����ˮ�������ᷢ���кͷ�Ӧ�����ŷ�Ӧ�Ľ��У�NaOHŨ����Сֱ����ȫ���ģ��������Ҳ����ֱ��û�д�����(2��) ����(4)����NaOH��Ũ��(�����)(2��) ����(5)��������ˮ������в��ֻӷ�(2��) |

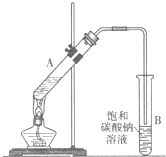 ������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ��
������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ���й����ݼ�����Ӧ��
| ���� | �Ҵ� | �������� | ���� | |
| �е�/�� | 118 | 78.3 | 77.1 | 34.5 |
| �ܽ��� | ������ˮ | ��������ˮ | �����ѻ��� | ����ˮ |
| Ũ���� |
| ��140 |
��ش��������⣺
��1���ڴ��Թ�A�����ӵ��Լ���6mL�Ҵ���4mL�����4mLŨ���ᣬ�������Լ�������˳������Ϊ
��2���Թ�B�е��ܽӽ�Һ��δ����Һ���µ�������
��3���ֶ��Թ�B�����������ֲ�Ʒ�����ᴿ���������£�
�ٽ��Թ�B�л��Һ������ת��
�����������ϲ�Һ���м�����ˮ�����ƣ������������ˮ�����Ƶ�Ŀ���ǣ�
�۽���������������Һ���������������ƿ�У�������������ռ�
��4������ɫ��ѧ�ĽǶȷ�����ʹ��Ũ������������������֮����Ҫ��
��5������ʱ����һ��ƺʹ���ʹ��������ɿڣ����÷���ʵ������Ļ�ѧ����ʽ���ͣ�
ʵ�鲽�裺
���Թ��м���8mLNaOH��Һ���ټ���2mL������������ֱ���������������ĸ߶ȣ��ٰ��Թܷ���70���ˮԡ�У�ÿ��1min����ȡ���������ã�������������¼ʣ������ĸ߶ȣ���Ѹ�ٷŻ�ˮԡ�м������ȣ���˷������У��ı�NaOH��ҺŨ�ȣ��ظ�ʵ�飮���ݼ�¼��
��������ˮ���ʣ��ĸ߶�/min
| ��� | c��NaOH��/mol?L-1 | ʱ��/min | |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 1 | 0.5 | 10.0 | 9.0 | 8.0 | 7.5 | 7.0 | 6.5 | 6.5 | 6.5 |
| 2 | 1.0 | 10.0 | 8.5 | 7.0 | 6.0 | 5.0 | 4.5 | 4.5 | 4.5 |
| 3 | 2.0 | 10.0 | 8.0 | 6.0 | 4.5 | 3.0 | 2.0 | 1.5 | 1.5 |
��1����������Ա�ʵ��ʱ��ÿ��ʵ�鶼������Ʋ����������
��2�������������ݣ��õ���������ˮ�����ʵĽ����ǣ�
��
����������ˮ�������ȿ������һ��ʱ���ﵽƽ��״̬��
��3�����ۢڵ����۽�����
��4����ʹ����������ȫˮ�⣬�ɲ�ȡ�Ĵ�ʩ��
��5����ͬѧ��Ϊ�б�Ҫ������ˮ����NaOH��Һ�ظ����飬�����ݽ�����������Ҫԭ����





 C2H5OC2H5+H2O
C2H5OC2H5+H2O