��Ŀ����
ʵ��������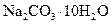 ��������
��������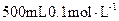 ��
��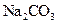 ��Һ����ش��������⡣
��Һ����ش��������⡣
��1��Ӧ��������ƽ��ȡ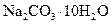 ����_______g��
����_______g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ�1g����ʹ�����룩����ƽƽ��ʱ��ʵ�ʳ�����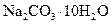 ������__________g��
������__________g��
��3����ʵ���õ�����Ҫ�����У�������ƽ����Ͳ���ձ�����������________��________��
��4�����������ʹ������Һ��Ũ�Ȳ�������Ӱ�죨�ƫ�ߡ�����ƫ�͡��������䡱������
a.�ܽ⾧���õ��ձ��Ͳ�����δϴ�ӡ�____________
b.����ʱ���ӿ̶��ߡ�____________
c.����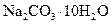 ������ʧȥ���ֽᾧˮ��____________
������ʧȥ���ֽᾧˮ��____________
��5����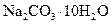 �����������
�����л������� ���壬���������Ƶ�
���壬���������Ƶ�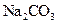 ��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱����
��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱����
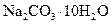 ��������
�������� ��
��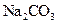 ��Һ����ش��������⡣
��Һ����ش��������⡣��1��Ӧ��������ƽ��ȡ
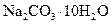 ����_______g��
����_______g����2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ�1g����ʹ�����룩����ƽƽ��ʱ��ʵ�ʳ�����
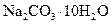 ������__________g��
������__________g����3����ʵ���õ�����Ҫ�����У�������ƽ����Ͳ���ձ�����������________��________��
��4�����������ʹ������Һ��Ũ�Ȳ�������Ӱ�죨�ƫ�ߡ�����ƫ�͡��������䡱������
a.�ܽ⾧���õ��ձ��Ͳ�����δϴ�ӡ�____________
b.����ʱ���ӿ̶��ߡ�____________
c.����
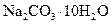 ������ʧȥ���ֽᾧˮ��____________
������ʧȥ���ֽᾧˮ��____________��5����
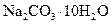 �����������
�����л�������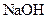 ���壬���������Ƶ�
���壬���������Ƶ�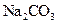 ��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱����
��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱������1��14.3
��2��13.7
��3����ͷ�ιܣ�500mL����ƿ
��4��a.ƫ�� b.ƫ�� c.ƫ��
��5��ƫ��
��2��13.7
��3����ͷ�ιܣ�500mL����ƿ
��4��a.ƫ�� b.ƫ�� c.ƫ��
��5��ƫ��
��

��ϰ��ϵ�д�
�����Ŀ
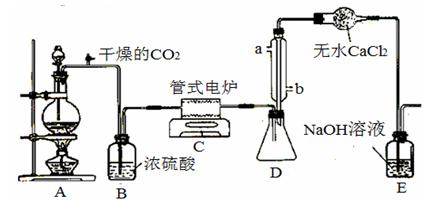
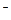 ��CO32-��OH
��CO32-��OH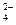 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩. L-1 H2SO4 b��0.01mol
L-1 H2SO4 b��0.01mol Ԥ������ͽ���
Ԥ������ͽ���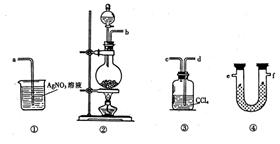
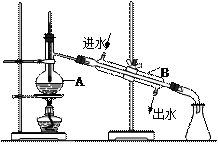
 ��B������������ ��
��B������������ ��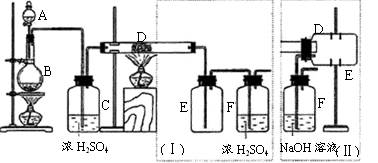
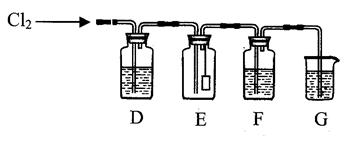
 ����ʽ ��
����ʽ ��