��Ŀ����
�����й���Һ������Ũ�ȵĹ�ϵʽ����ȷ����
| A��25��ʱ��0.1 mol��L��1pH=4.5��NaHC2O4��Һ��c(HC2O4-)��c(H2C2O4)��c(C2O42��) |
| B����0.2 mol��L��1NaHCO3��Һ�м�������0.1 mol��L��1NaOH��Һ�� c(H��) ��c(Na��)��c(OH��)��2c(CO32��) ��c(HCO3��) |
| C�������£�NH4Cl��NH3��H2O�Ļ����Һ[pH=7��c(Cl��)=0.1mol/L]�У� c(Cl��) > c(NH4+) > c(OH��) = c(H+) |
| D��Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COONa��CH3COOH�����Һ�� |
BD
����

��ϰ��ϵ�д�
�����Ŀ

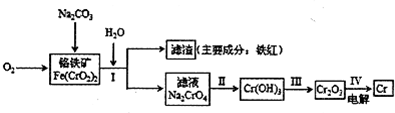
 Cr2O72-+H2O
Cr2O72-+H2O



