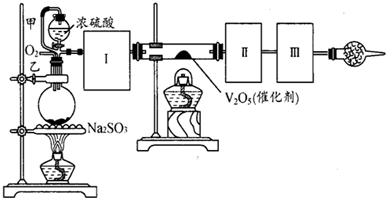
��Ŀ����
����ͼװ�ÿ��Խ��вⶨSO2ת����SO3��ת���ʵ�ʵ�飮��֪SO3���۵���16��8�棬�е���445.8�森��֪����װ�������漰��Ӧ�Ļ�ѧ����ʽΪ��Na2SO3��s��+H2SO4��98%���TNa2SO4+H2O+SO2��
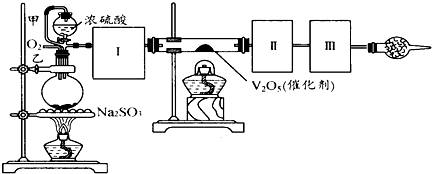
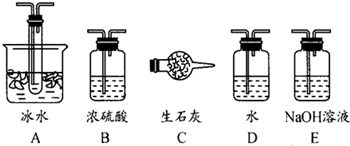
��1������ʵ����Ҫ��Ӧ���ڢ����Ӻ��ʵ�װ�ã������ͼA��Eװ����ѡ�����ʺ�װ�ò����������������Ŀո��У�
�����ӵ�װ�÷ֱ���

��2�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ�����ڼ��ȴ�����μ�Ũ�����˳���У�Ӧ��ȡ�IJ�����
��3����SO2ͨ�뺬1.5mol�������Һ�У�������һ��ǿ���һ�����������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ
��4����amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ����װ�â�������bg����ʵ����SO2��ת����Ϊ
%���ú�a��b�Ĵ���ʽ��д��
��5��β�����θ���ܵ�������

��1������ʵ����Ҫ��Ӧ���ڢ����Ӻ��ʵ�װ�ã������ͼA��Eװ����ѡ�����ʺ�װ�ò����������������Ŀո��У�
�����ӵ�װ�÷ֱ���
B
B
��A
A
��E����C��
E����C��
��
��2�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ�����ڼ��ȴ�����μ�Ũ�����˳���У�Ӧ��ȡ�IJ�����
�ȼ��ȴ����ٵ���Ũ����
�ȼ��ȴ����ٵ���Ũ����
����3����SO2ͨ�뺬1.5mol�������Һ�У�������һ��ǿ���һ�����������1.5��6.02��1023������ת��ʱ���÷�Ӧ�Ļ�ѧ����ʽ
SO2+2HClO3=H2SO4+2ClO2
SO2+2HClO3=H2SO4+2ClO2
��4����amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ����װ�â�������bg����ʵ����SO2��ת����Ϊ
| 1600a-25b |
| 16a |
| 1600a-25b |
| 16a |
��5��β�����θ���ܵ�������
��ֹ�����е�CO2��ˮ�������ţ�������
��ֹ�����е�CO2��ˮ�������ţ�������
����������1��������Ũ��������������������壻SO3���۵���16��8�棬�ϵͣ������ñ�ˮ����������������ü�ʯ�һ�������������Һ����β��������
��2���ȼ��ȴ����ٵ���Ũ���ᣬ�ܱ�֤�����Ķ��������ܶ��ת��Ϊ��������
��3��������������ԣ�������Ԫ�صĻ��ϼ�Ϊ+5�ۣ�����ת�Ƶĵ��������жϲ�������Ԫ�صļ�̬��
��4��������ԭ���غ����ȷ�����������ת���ʣ�
��5�������е�CO2��ˮ��������ŲⶨSO2ת����SO3��ת���ʣ�
��2���ȼ��ȴ����ٵ���Ũ���ᣬ�ܱ�֤�����Ķ��������ܶ��ת��Ϊ��������
��3��������������ԣ�������Ԫ�صĻ��ϼ�Ϊ+5�ۣ�����ת�Ƶĵ��������жϲ�������Ԫ�صļ�̬��
��4��������ԭ���غ����ȷ�����������ת���ʣ�
��5�������е�CO2��ˮ��������ŲⶨSO2ת����SO3��ת���ʣ�
����⣺��1����װ�ñ���Ҫ�Զ���������и��������Ũ��������������������壻SO3���۵���16��8�棬�����ñ�ˮ�������������δ��Ӧ���Ķ�������Կ����������Ⱦ�������ü�ʯ�һ�������������Һ������β���������ʴ�Ϊ��B�� A��E����C����
��2��Ϊ��֤�����Ķ��������ܶ��ת��Ϊ��������Ӧ�ȼ��ȴ����ٵ���Ũ���ᣬ�ʴ�Ϊ���ȼ��ȴ����ٵ���Ũ���
��3��������������ԣ�������Ԫ�صĻ��ϼ�Ϊ+5�ۣ���1.5��6.02��1023����1.5mol����ת��ʱ��1.5mol��������Ԫ�صĻ��ϼ�Ӧ��+5�۽���+4�ۣ����Բ�������Ԫ���Զ������ȵ���ʽ���ڣ�������������Ϊ���ᣬ�ʴ�Ϊ��SO2+2HClO3=H2SO4+2ClO2��
��4��������ԭ���غ㣬Na2SO3��SO2��SO3��amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ��Ӧ�����������������Ϊ64ag�����װ�â�������bg����Ϊʣ�������������������Զ��������ת���ʦ�=
��100%=
%���ʴ�Ϊ��
��
��5�������е�CO2��ˮ��������ŲⶨSO2ת����SO3��ת���ʣ�����Ҫ��һ������װ�ã��ʴ�Ϊ����ֹ�����е�CO2��ˮ�������ţ������
��2��Ϊ��֤�����Ķ��������ܶ��ת��Ϊ��������Ӧ�ȼ��ȴ����ٵ���Ũ���ᣬ�ʴ�Ϊ���ȼ��ȴ����ٵ���Ũ���
��3��������������ԣ�������Ԫ�صĻ��ϼ�Ϊ+5�ۣ���1.5��6.02��1023����1.5mol����ת��ʱ��1.5mol��������Ԫ�صĻ��ϼ�Ӧ��+5�۽���+4�ۣ����Բ�������Ԫ���Զ������ȵ���ʽ���ڣ�������������Ϊ���ᣬ�ʴ�Ϊ��SO2+2HClO3=H2SO4+2ClO2��
��4��������ԭ���غ㣬Na2SO3��SO2��SO3��amolNa2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ��Ӧ�����������������Ϊ64ag�����װ�â�������bg����Ϊʣ�������������������Զ��������ת���ʦ�=
| 64a-b |
| 64 |
| 1600a-25b |
| 16a |
| 1600a-25b |
| 16a |
��5�������е�CO2��ˮ��������ŲⶨSO2ת����SO3��ת���ʣ�����Ҫ��һ������װ�ã��ʴ�Ϊ����ֹ�����е�CO2��ˮ�������ţ������
������������һ���йض����������ȡ�Լ����ʵ�ʵ����Ŀ���ۺ��Խ�ǿ���ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 Na2SO4+SO2��+H2O��ע��80%H2SO4����Ũ��������ԣ�
Na2SO4+SO2��+H2O��ע��80%H2SO4����Ũ��������ԣ�