��Ŀ����
A��B��Ϊ���ε�ˮ��Һ��A�����ԣ�B�ʼ��Բ����������ԡ�����Ϊ���ʵ�鲽���ʵ������
������������⣺
(1)д��A��B��C�Ļ�ѧʽ��A_____________��B_____________��C_____________��
(2)����д��A��D��D��E(E�к���ij+5��Ԫ�صĺ����������)�����ӷ���ʽ��_______________________________________��_______________________________________��
(3)��SO2����ͨ��D��Һ��D��Һ��Ϊ��ɫ�����������ᡣд����Ӧ�����ӷ���ʽ��____________________________________________________________________��
(4)д����F��H�Ļ�ѧ����ʽ��__________________________________________________��
�������������ͻ�ƿ�Ϊ�������ʵ���ɫ���ɴ˿�֪Ϊ±�ص��ʼ��仯����֮����ת�䣬����B�ʼ��Բ����������Լ�H�Ǻ�B����Һ�����Ƴ�AΪNaI��BΪNaClO��CΪAgI��DΪI2��(2)��NaClO����ǿ�����ԣ��ɰ�I2����ΪNaIO3��(3)I2�ɰ�SO2����Ϊ![]() ��(4)FHΪCl2��NaOH�ķ�Ӧ��
��(4)FHΪCl2��NaOH�ķ�Ӧ��
�𰸣�(1)NaI NaClO AgI
(2)2I-+ClO-+H2O![]() I2+Cl-+2OH-
I2+Cl-+2OH-
I2+5ClO-+2OH-![]() 2
2![]() +5Cl-+H2O
+5Cl-+H2O
(3)I2+SO2+2H2O![]() 2I-+
2I-+![]() +4H+
+4H+
(4)Cl2+2NaOH![]() NaCl+NaClO+H2O
NaCl+NaClO+H2O


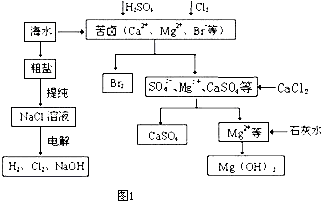
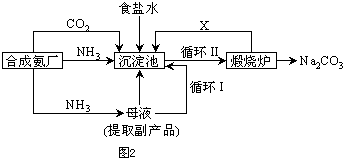
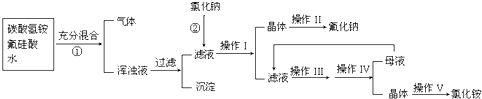
 �����ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl(����)����BaCl2����3���Լ����ӵĺ���˳����_________(�����)��
�����ʣ����뾫�ƺ���ܹ����ʹ�á�����ʱ����������ˮ���˺�Ҫ������Լ��ֱ�Ϊ��Na2CO3����HCl(����)����BaCl2����3���Լ����ӵĺ���˳����_________(�����)��
