��Ŀ����
����Ŀ��ijѧϰС�鰴����ʵ������̽�������е⺬���IJⶨ�͵����ȡ��
ʵ��һ �⺬���IJⶨ
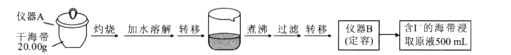
ȡ0.0100 mol/L�� AgNO3����Һװ��ζ��ܣ�ȡ100.00 mL ������ȡԭҺ���ζ��أ��õ��Ƶζ����ⶨ�⺬������õĵ綯�ƣ�E)��ӳ��Һ��c(I-)�ı仯�������������±���
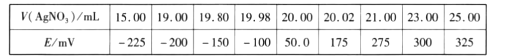
�ش��������⣺
(1)ʵ����������������___________�����������ƣ�����ɵġ�
(2)�����־������������������̣�__________________________________
(3)���ݱ��������жϵζ��յ�ʱ��ȥAgNO3��Һ�����Ϊ___________mL,����ú����е�������ٷֺ���Ϊ_______________________����
ʵ��� �����ȡ
���ƺ�����ȡԭҺ���ס�������ʵ�鷽�����£�
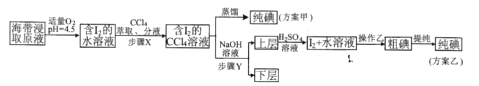
��֪��3I2+6NaOH=5NaI+NaIO3+3H2O
(4)������O2�����ܴ���O2��������ʶ�Ӧ�ĵ���ʽΪ_________________
(5)��Ҫ�ⶨ��I2+ˮ��Һ���е�ĺ���������ѡ��______________��ָʾ������ Na2S2O3��Һ�ζ����ζ��յ��������____________________��
(6)���õ���I2+ˮ��Һ��ʱ����������Һ���࣬��Na2S2O3��Һ�ζ�ʱ��������Ե�������������ԭ��Ϊ_________________�������ӷ���ʽ��ʾ����
���𰸡����� ����ƿ������ˮ���̶�����1-2cm�������ý�ͷ�ιܵμ�����ˮ����Һ����ʹ���̶������� 20.00 0.635 ![]() ������Һ �μ����һ��Na2S2O3��Һʱ����ƿ�ڵ���Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ ��2H++ S2O32-=S��+SO2��+H2O
������Һ �μ����һ��Na2S2O3��Һʱ����ƿ�ڵ���Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ ��2H++ S2O32-=S��+SO2��+H2O
��������
ʵ��һ(1)ʵ������������һ���������н��У�
(2)��������һ�����ʵ���Ũ����Һ���ݲ���������
(3)������������Һ�ζ������ӣ���ϵ綯�Ƶı仯�жϵζ��յ㣬��������⺬����
ʵ���(4) �����е����ӵĺ�������ԭҺͨ����������������������Ϊ�ⵥ�ʣ����������������������������Ҳ������µ����ʣ�
(5)�ⵥ�����������
(6)���������£�Na2S2O3�����ᷢ���绯��Ӧ��
ʵ��һ(1)ʵ������������һ���������н��У���������յ���������Ϊ������
(2)��������һ�����ʵ���Ũ����Һ���ݲ���Ϊ������������ƿ������ˮ���̶�����1-2cm�������ý�ͷ�ιܵμ�����ˮ����Һ����ʹ���̶������У�
(3)������������Һ�ζ������ӣ����ݱ����н�ϵ綯�Ƶı仯�ɵã���������������Һ�����Ϊ19.98~20.02mL֮��ʱ���綯�Ʒ���ͻ�䣬ȡ�м�ֵΪ20.00mL�����İٷֺ���Ϊ![]() ��100%=0.635%��
��100%=0.635%��
ʵ���(4)�����е����ӵĺ�������ԭҺͨ����������������������Ϊ�ⵥ�ʣ����������������������������Ҳ������µ����ʣ�����ѡ��˫��ˮ�������������������ӣ�˫��ˮ�ĵ���ʽΪ![]() ��
��
(5)�ⵥ�������۱�������ζ��������õ�����Һ��ָʾ�����ζ��յ�ʱ���ⵥ�ʱ�Na2S2O3��Һȫ��ת��Ϊ�����ӣ�����Ϊ���μ����һ��Na2S2O3��Һʱ����ƿ�ڵ���Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
(6)���������£�Na2S2O3�����ᷢ���绯��Ӧ�����ӷ�ӦΪ��2H++ S2O32-=S��+SO2��+H2O��

����Ŀ����ҵ���ú��̷���(��Ҫ�ɷ�ΪMnO2����������Fe2O3��Al2O3��CuO��CaO��)����������������ϴ������Ʊ�MnSO4��������ͼ��ʾ��

��֪��25��ʱ����������������ܶȻ�����(Ksp)�����ʾ��
�������� | Al(OH)3 | Fe(OH)3 | Cu(OH)2 | Mn(OH)2 |
Ksp | 1.3��10-33 | 4.0��10-38 | 2.2��10-20 | 1.9��10-14 |
�ش��������⣺
(1)����1�Ļ�ѧʽΪ____��
(2)�����£�����pHΪ5����ͨ������˵����ʱAl3+��Fe3+�ѳ�����ȫ��____��
(3)(NH4)2S�ĵ���ʽΪ____����������ʱ������(NH4)2S������Ϊ____��
(4)���ữ����ԭ���У�����������������ԭ��Ӧ�����ӷ���ʽΪ____��
(5)��֪����Һ3�г�MnSO4�⣬����������(NH4)2SO4��(NH4)2SO4��MnSO4���ܽ��������ͼ��ʾ���ݴ��жϣ���������ӦΪ����Ũ����____��ϴ�ӡ����

(6)��ҵ�Ͽ��õ������MnSO4��Һ�ķ����Ʊ�MnO2����������ӦʽΪ____��





