��Ŀ����
��2012?�ӱ���ģ�⣩�±���ʵ�����Ʊ�������й����ݣ�
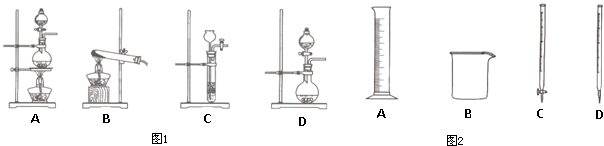
��1���ӷ�Ӧԭ����������ѡ����ʵ�����������ʵ�ֵ���
��2�����ݱ���������������ʵ��ԭ������ͼ1װ����ѡ����ʵ����巢��װ�ÿ�����
��3�����ñ��е�ʵ��ԭ���Ʊ�NH3����ѧ����ʽΪ��
��4���Ʊ�Cl2����10mol?L-1������100mL������12mol?L-1�����������ƣ�
��Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ��ͼ2��
�����ƹ����У���ͼ1���������⣬����Ҫ��������
��5����ӷ�Ӧԭ������������Ӧ��ľۼ�״̬������д������ͼ1��C����װ���Ʊ���3������Ļ�ѧʽ��
| ��� | ʵ������ | ʵ��ԭ�� | ���巢��װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |

��1���ӷ�Ӧԭ����������ѡ����ʵ�����������ʵ�ֵ���
��
��
��д��ţ������Բ�ͬ�������������������
��
��д��ţ�����2�����ݱ���������������ʵ��ԭ������ͼ1װ����ѡ����ʵ����巢��װ�ÿ�����
A��D
A��D
����3�����ñ��е�ʵ��ԭ���Ʊ�NH3����ѧ����ʽΪ��
2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
| ||
2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
��
| ||
��4���Ʊ�Cl2����10mol?L-1������100mL������12mol?L-1�����������ƣ�
��Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ��ͼ2��
C
C
������ĸ���������ƹ����У���ͼ1���������⣬����Ҫ��������
100mL����ƿ������������ͷ�ι�
100mL����ƿ������������ͷ�ι�
�����������ƣ�����5����ӷ�Ӧԭ������������Ӧ��ľۼ�״̬������д������ͼ1��C����װ���Ʊ���3������Ļ�ѧʽ��
CO2��H2��H2S
CO2��H2��H2S
����������1���������������Ʊ����Լ��ͱ仯��������Ҫ�����������Ʊ������������Ǽ������壻
��2�������Ʊ�������Լ�״̬����Ӧ���������жϣ�����+Һ������������װ��ѡ��A������+��������������װ��ѡ��B������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC������+Һ�岻�����������װ��ΪD��
��3���Ȼ�狀��������Ʒ�Ӧ�õ�������
��4����������Ƶľ�ȷ�ȣ���ȡŨ�����������ʽ�ζ�����ȡ��
������������Һ��ʵ�鲽�����ʹ�õ��������ƣ�
��5������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC��
��2�������Ʊ�������Լ�״̬����Ӧ���������жϣ�����+Һ������������װ��ѡ��A������+��������������װ��ѡ��B������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC������+Һ�岻�����������װ��ΪD��
��3���Ȼ�狀��������Ʒ�Ӧ�õ�������
��4����������Ƶľ�ȷ�ȣ���ȡŨ�����������ʽ�ζ�����ȡ��
������������Һ��ʵ�鲽�����ʹ�õ��������ƣ�
��5������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC��
����⣺��1�����ù�������ֽ��������������Ȼ�狀��������ƹ�����ȷ������ֽⷴӦ�ư��������ö�����������Ũ������������������Ҫ�����������Ʊ����������а����Ǽ������廯����������������嵥�ʣ����������������嵥�ʣ��ӷ�Ӧԭ���������Բ�ͬ������������������Ʊ��������õ��Ǹ��ֽⷴӦ���ʴ�Ϊ���ۣ��ڣ�
��2�������Ʊ�������Լ�״̬����Ӧ���������жϣ�����+Һ������������װ��ѡ��A������+��������������װ��ѡ��B������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC������+Һ�岻�����������װ��ΪD�����ö�����������Ũ�������������ѡ��A�����ø�����غ�Ũ������ȷ�Ӧ��������ѡ��D���ʴ�Ϊ��A��D��
��3���Ȼ�狀��������Ʒ�Ӧ�õ���������ѧ����ʽΪ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
��4����Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ����ʽ�ζ��ܼ�����ȡ���ʴ�Ϊ��C��
�����ƹ����У�����ͼ���������⣬����Ҫ���������ܽ���Ҫ���ձ���������Һ�õ���100ml������ƿ���ܽ�������õ��IJ������������õ��Ľ�ͷ�ιܣ��ʴ�Ϊ��100mL����ƿ������������ͷ�ιܣ�
��5������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC����CO2��H2��H2S���ʴ�Ϊ��CO2��H2��H2S��
��2�������Ʊ�������Լ�״̬����Ӧ���������жϣ�����+Һ������������װ��ѡ��A������+��������������װ��ѡ��B������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC������+Һ�岻�����������װ��ΪD�����ö�����������Ũ�������������ѡ��A�����ø�����غ�Ũ������ȷ�Ӧ��������ѡ��D���ʴ�Ϊ��A��D��
��3���Ȼ�狀��������Ʒ�Ӧ�õ���������ѧ����ʽΪ��2NH4Cl+Ca��OH��2
| ||
| ||
��4����Ϊ������Ƶľ�ȷ�ȣ���ȡŨ�����������ѡ����ʽ�ζ��ܼ�����ȡ���ʴ�Ϊ��C��
�����ƹ����У�����ͼ���������⣬����Ҫ���������ܽ���Ҫ���ձ���������Һ�õ���100ml������ƿ���ܽ�������õ��IJ������������õ��Ľ�ͷ�ιܣ��ʴ�Ϊ��100mL����ƿ������������ͷ�ιܣ�
��5������+Һ�岻����������װ������ʱ������ʱֹͣ��װ��ѡC����CO2��H2��H2S���ʴ�Ϊ��CO2��H2��H2S��
���������⿼�������Ʊ�װ��ѡ���ռ�����Һ�����Ƶȣ���ȷ��װ�õ���;�ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д� ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д�
�����Ŀ