��Ŀ����
�ɼ������ӻ�������ɵĻ����������������е������֣�K����NH4+��Mg2����Ba2����Cl����NO2-��SO42-��CO32-�����û��������ˮ��ó�����Һ����ȡ4��100 mL����Һ�ֱ��������ʵ�飺
| ʵ�� ��� | ʵ������ | ʵ���� |
| A | ��AgNO3��Һ | �а�ɫ�������� |
| B | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����) |
| C | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�����������Ϊ6.27 g���ڶ��γ�����������Ϊ2.33 g |
| D | ������KMnO4������Һ | KMnO4��Һ��ɫ |
�Իش��������⣺
(1)����ʵ��a�ж�Cl���Ƿ����________(�һ�����ڡ�����һ�������ڡ�����ȷ����)��
(2)�û������һ�������ڵ�������_______________________________��
(3)��д��ʵ��b�з�����Ӧ�����ӷ���ʽ________��
��д��ʵ��d��ʹKMnO4������Һ��ɫ�����ӷ���ʽ________________��
(4)��Һ��һ�����ڵ������Ӽ������ʵ���Ũ��Ϊ(�ɲ�����)��
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| | |
| | |
| | |
(5)��������Ƿ����K����________���жϵ�������__________________��
(1)����ȷ����(2)Ba2����Mg2��
(3)��NH4+��OH�� NH3����H2O
NH3����H2O
��5NO2-��2MnO4-��6H��=5NO3-��2Mn2����3H2O
(4)�����ӷ��� ���ʵ���Ũ��(mol��L��1) SO42- 0.1 CO32- 0.2 NO2- ��
(5)���ڡ�ͨ��ʵ���֪��Һ�п϶����ڵ�������NH4+��CO32-��SO42-��NO2-�������㣬NH4+�����ʵ���Ũ��Ϊ0.5 mol��L��1��CO32-��SO42-�����ʵ���Ũ�ȷֱ�Ϊ0.2 mol��L��1��0.1 mol��L��1�����ݵ���غ��K��һ������
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(14��)����п�������ᡢ�´ɡ����¡�ҽҩ�����ӡ���ѧ�ȹ�ҵ����Ҫԭ�ϡ�����
��п��ƷΪԭ���Ʊ���������п�����������������£�
��1��.��������õ���������Һ�к���Zn2����SO42����������Fe2����Cu2���� ����Mn2����
����Mn2����
���ʡ�����A�������ǵ�����Һ��pH��5 4������A���ѡ��________��
| A��NH3.H2O | B��Na2CO3 | C��H2SO4 | D��ZnO |

���¶���
 ��
����2�� KMnO4�������dz�ȥMn2����Fe2������KMnO4��Mn2����Ӧ�����ӷ���ʽΪ_____________________________________������Һ��
 ������1
������1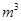 ����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���
����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣�����3������Cu2����
 2���������û���Ӧ��ȥ��������B��_________��
2���������û���Ӧ��ȥ��������B��_________����4�������յij�����
 �����ɸó����Ļ�ѧ����ʽΪ________��
�����ɸó����Ļ�ѧ����ʽΪ________����μ���ó����Ƿ�ϴ����________________________________��
��һ��ɫ������Һ��Ҫȷ���Ƿ����������ӣ�H+��K����Mg2����Al3����Fe2����Ba2����NO3����SO42����Cl����I����HCO3����ȡ����Һʵ�����£�
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ���ɫ |
| (2)ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
��1����Һ�п϶����ڵ������� ����Һ�п϶������ڵ������� ��
��2��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ�������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ��������)�� ��
A��B��C��D��E��F��G�������ʼ������ͼ��ʾ��ת����ϵ������A��B��D��G����ͬ��Ԫ�ء�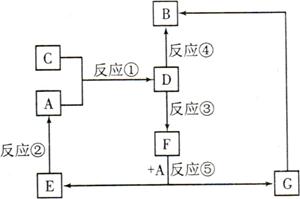
��֪��
AΪ�������ʣ�BΪ���ɫ���壬EΪ�ܶ���С�����壬GΪdz��ɫ����Һ��
D��ˮ��ҺΪ��ɫ��Һ��������������Һ��Ӧ���ɲ�����ϡ����İ�ɫ������
��ˮ��Һ��D�ܽ�ij����������ΪF��F�Ǻ�������Ԫ�صĻ����
��ش��������⣺
��1������C���ʵ�Ԫ�������ڱ��е�λ���� ���ڶ���������Ԫ���У���Ԫ����������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)��
��2��D��ˮ��Һ�� �ԣ��������ӷ���ʽ����ԭ�� ��
��3��������Ӧ�������û���Ӧ���� (�����)��
��4����Ӧ��(��D��ij������������ΪF)�����ӷ���ʽ�� ��
��5��������C��������ʵ�顣��֪������Ӧ�����У�ÿ����0.1mol KI��ת�Ƶĵ�����ԼΪ3.612��1023�����밴��Ҫ����գ�
| ʵ�鲽�� | ʵ������ | д���ӷ���ʽ |
| ����������ͨ�����KI��Һ | ��Һ������ ɫ | |
| ����ͨ������ | ��Һ�����ɫ | |
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
��1����Һ��һ�����ڵ�������______________����Һ�п϶������ڵ�������________________________��
��2��Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ���������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ����)________________________________________��
��3��д��ʵ��������з�Ӧ�����ӷ���ʽ��______________________________��


