��Ŀ����
|
�����Ƕ�Ԫ��ǿ�ᣬ����������Һ�����ԣ������£���10 mL��0.01 mol��L��1��NaHC2O4��Һ�еμ�0.01 mol��L��1��NaOH��Һ������NaOH��Һ��������ӣ���Һ������Ũ�ȹ�ϵ��ȷ���� | |
| [����] | |
A�� |
V(NaOH)��0ʱ��c(H+)��1��10��2 mol��L��1 |
B�� |
V(NaOH)��10 mLʱ�������ܴ���c(Na+)��2c(C2O42��)��c(HC2O4��) |
C�� |
V(NaOH)��10 mLʱ��c(H+)��1��10��7 mol/L |
D�� |
V(NaOH)��10 mLʱ��c(Na+)��c(C2O42��)��c(HC2O4��) |
�𰸣�B

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�����Ƕ�Ԫ��ǿ�ᣬ����������Һ�����ԡ������£���10 mL 0.01 mol��L NaHC2O4��Һ�еμ�0.01 mol��L NaOH��Һ������NaOH��Һ��������ӣ���Һ������Ũ�ȹ�ϵ��ȷ����
| A��V��NaOH��="=" 0ʱ��c��H+��W��="=" 1 �� 10��2 mol��L |
B��V��NaOH��< 10 mLʱ�������ܴ���c��Na����="=" 2 c��C2O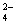 ��+ c��HC2O ��+ c��HC2O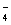 �� �� |
| C��V��NaOH��="=" 10 mLʱ��c��H+��W��="=" 1 �� 10��7mol��L |
D��V��NaOH��> 10 mLʱ��c��Na����> c��C2O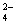 ��>c��HC2O ��>c��HC2O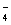 �� �� |