��Ŀ����
ij��ɫ��Һ�������п��ܴ��ڵ��������£�Na+��Ag+��Ba2+��Al3+��AlO2-��S2-��CO32-��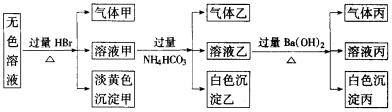
(1)��������________��
���ɼ����ӷ���ʽΪ________________________��
(2)��������________��
����Һ�������ҵ����ӷ���ʽΪ__________________��
(3)��������________�������һ��ѧʵ����ȷ����ɷ֣���____________________��
(4)����׳ɷ����ļ��ֿ��ܣ���______________________________________��
(5)���Կ϶����ڵ�������________________________��һ��������_____________��
�𰸣�
������
������
| (1)S,2S2-+SO32-+6H+====3S��+3H2O
(2)Al(OH)3,Al3++3HCO3-====Al(OH)3��+3CO2�� (3)BaCO3��BaCO3��BaSO4,����ϡHCl����������ȫ�ܽ�Ϊǰ�ߣ������ܽ��Ϊ���� (4)H2S��SO2��CO2 (5)Na+��AlO2-��S2-��SO32-,Ag+��Ba2+��Al3+
|

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
