��Ŀ����
ȡ3.40 gֻ���ǻ����������������ŵ�Һ̬���Ͷ�Ԫ��������5.00 L�����У�����ȼ������ȫȼ�ա���Ӧ�������������0.56 L�������徭����NaOH��Һ���գ�����ּ���2.80 L(����������ڱ�״���²ⶨ)��
(1)3.40 g����C��H��O���ʵ����ֱ�ΪC_________mol,H_________mol,O_________mol,�ô���C��H��O��ԭ����֮��Ϊ_________��
(2)�����ϱ�ֵ�ܷ�ȷ���ô��Ļ�ѧʽ��_________����ԭ����______________________��
(3)������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��__________________��
(1)0.125��0.300��0.100��5��12��4��
(2)���� ��Ϊ��ʵ��ʽ����ԭ�Ӹ����ѱ��ͣ�����ʵ��ʽ����ѧʽ��(3)C(CH2OH)4
��������
�����������(1)�����⣬���徭����NaOH���գ��������2.80 L���������ΪCO2���������3.40 g����C�����ʵ���Ϊ��n(C)=n(CO2)=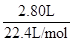 ="0.125" mol,����CO2����O2���������CO2�������ҲΪ2.80 L������ΪҺ̬����5.00 L O2ȼ�գ��������0.56 L���ʻ���0.56 L O2��������ˮ���������ر�ע����ǣ����ɵ�H2O�л��в�����ԭ�������ڴ�����˲��ܸ���0.56 L O2������H2O������Ҫ���ݷ�Ӧǰ���������������㴼��H�����ʵ������μӷ�Ӧ��V(O2)="0.56" L+2.80
L="3.36" L,m(O2)=
="0.125" mol,����CO2����O2���������CO2�������ҲΪ2.80 L������ΪҺ̬����5.00 L O2ȼ�գ��������0.56 L���ʻ���0.56 L O2��������ˮ���������ر�ע����ǣ����ɵ�H2O�л��в�����ԭ�������ڴ�����˲��ܸ���0.56 L O2������H2O������Ҫ���ݷ�Ӧǰ���������������㴼��H�����ʵ������μӷ�Ӧ��V(O2)="0.56" L+2.80
L="3.36" L,m(O2)=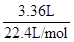 ��32 g/mol="4.80" g,m(H2O)="3.40g+4.80g-0.125��44"
g="2.70" g��3.40 g����n(H)=2��
��32 g/mol="4.80" g,m(H2O)="3.40g+4.80g-0.125��44"
g="2.70" g��3.40 g����n(H)=2��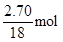 ="0.300" mol,3.40g����
="0.300" mol,3.40g���� ��
��
��n(C)��n(H)��n(O)="0.125" mol��0.300 mol��0.100mol=5��12��4���ʸô����ʽΪC5H12O4��
(2)��H�ѱ��ͣ��������������Ƿ���ʽ��
(3)�ɷ���ʽC5H12O4֪���ö�Ԫ��Ϊ��Ԫ����������ĿҪ������ṹ��ʽΪ��C(CH2OH4��
���㣺�����йط���ʽ�ͽṹʽȷ�����йؼ���
�������������е��Ѷȵ����⣬�����ۺ���ǿ���������С�ע�ػ���֪ʶ�Ĺ��̣����������������ͽ��ⷽ����ѵ����ָ��������������ѧ������˼ά�����ʹ���˼ά���������ѧ���������⡢������û���֪ʶ���ʵ�������������Ҳ���������ѧ���Ĺ淶����������Ӧ��������

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�