��Ŀ����
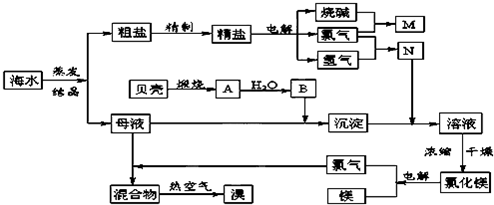
�ִ���ҵ����Ҫ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ȡH2��Cl2��NaOH����ش��������⣺
��1���ڵ������У����Դ���������ĵ缫���������ĵ缫��ӦʽΪ____________��
��2�����֮ǰʳ��ˮ��Ҫ���ƣ�Ŀ���dz�ȥ�����е�Ca2+��Mg2+��SO42-���������ӣ�ʹ�õ��Լ��У�a.Na2CO3��Һ��b.Ba(OH)2��Һ��c.ϡ���ᣬ������ļ���˳��Ϊ____________(���Լ����)��
��3��������ݻ�Ϊ10 L�����ӽ���Ĥ�����У�1 min�������ɲ���11.2 L(��״����)Cl2����ʱ��Һ��
pH��(����Һ������ֲ���)____________��
��1���ڵ������У����Դ���������ĵ缫���������ĵ缫��ӦʽΪ____________��
��2�����֮ǰʳ��ˮ��Ҫ���ƣ�Ŀ���dz�ȥ�����е�Ca2+��Mg2+��SO42-���������ӣ�ʹ�õ��Լ��У�a.Na2CO3��Һ��b.Ba(OH)2��Һ��c.ϡ���ᣬ������ļ���˳��Ϊ____________(���Լ����)��
��3��������ݻ�Ϊ10 L�����ӽ���Ĥ�����У�1 min�������ɲ���11.2 L(��״����)Cl2����ʱ��Һ��
pH��(����Һ������ֲ���)____________��
��1��2Cl--2e-==Cl2��
��2��bac��
��3��13
��2��bac��
��3��13

��ϰ��ϵ�д�
�����Ŀ

 �������һ���װ������ͼ�������ӽ���ĤA��B�����۷�ΪI��II��
�������һ���װ������ͼ�������ӽ���ĤA��B�����۷�ΪI��II��  �������һ���װ������ͼ�������ӽ���ĤA��B�����۷�ΪI��II��
�������һ���װ������ͼ�������ӽ���ĤA��B�����۷�ΪI��II��