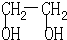��Ŀ����
ij�л�����C��H��O����Ԫ����ɣ������л���3g���ȼ������0.15mol������̼��3.6gˮ����֪���л���������ܶ�Ϊ2.68 g/L������ɱ�״���������л�����������Ʒ�Ӧ����
��1��ͨ������������л���ķ���ʽΪ ��
��2��д�����л���Ľṹ��ʽΪ �� ��
��3��ȡ3g���л���������Ʒ�Ӧ������H2�����Ϊ ������£�
��4�������л�����Ũ���Ṳ�ȿ��Ƶ�ϩ������д����Ӧ����ʽ
��������
�����������1�����л���������ܶ�Ϊ2.68 g/L������ɱ�״����
����л������Է���������2.68��22.4��60
��3g�л�������ʵ�����0.05mol
����ˮ��CO2�����ʵ����ֱ���0.2mol��0.15mol
�����ԭ���غ��֪
�л�����̼��ԭ�ӵ������ֱ���0.15mol��12g/mol��1.8g��0.4g
���Ը��������غ㶨�ɿ�֪
�л�������ԭ�ӵ�������3g��1.8g��0.4g��0.8g
���е���ԭ�ӵ����ʵ�����0.05mol
���Ը��л�����C��H��O����ԭ�ӵĸ���֮����3:8:1
����̼ԭ���Ѿ��ﵽ����״̬
�Ҵ����л���ķ���ʽ����C3H8O
��2�����л�����������Ʒ�Ӧ������ݻ�ѧʽ��֪�����л����Ǵ��࣬��ʾ���࣬���Խṹ��ʽ��CH3CH2CH2OH��CH3CHOHCH3
��3��3g�л�������ʵ�����0.05mol
�������ԭ���غ��֪
�������������ʵ�����0.05mol��2��0.025mol
���Ա�״���������������0.025mol��22.4/L��0.56L
��4���������Է�����ȥ��Ӧ���ɱ�ϩ����Ӧ�Ļ�ѧ����ʽ��CH3CH2CH2OH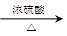 CH3CH=CH2����H2O��CH3CHOHCH3
CH3CH=CH2����H2O��CH3CHOHCH3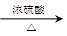 CH2=CHCH3����H2O��
CH2=CHCH3����H2O��
���㣺�����л��ﻯѧʽ���ṹ��ʽ���жϡ������Լ�����ʽ����д
�������������е��Ѷȵ����⣬���������ǿ������������������ѵ��������������ѧ���������������ͷ�ɢ˼ά������Ҳ����������ѧ���Ĺ淶�������������ѧ���������⡢��������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�