��Ŀ����
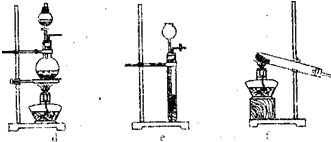
��ͼ��ʵ������ȡNH3��װ�ú�ѡ�õ��Լ�������Ҫ��ش�(1)ʵ�����Ʊ������NH3������ͼ����ʾװ�ûش�

�ٷ�Ӧ�Ļ�ѧ����ʽΪ___________��ʵ���в�ѡ��NaOH�Ʊ�NH3��ԭ����______________ ______________________________________��װ�����ռ�NH3���Թܿڷ������ŵ�������___________________________________________________________________��
����a��c֮�䰲װb���Ƿ��б�Ҫ?___________(��С���û�С�)�������С���Ӧװ��___________�Լ���(����û�С����ɲ������)����û�С���������_______________________________________��
(2)ʵ������������ȡ����NH3������ͼ����ʾװ�ûش�
���û�ѧ����ʽ��ʾ��Ũ��ˮ����CaO���д���NH3�ݳ���ԭ��_______________________��
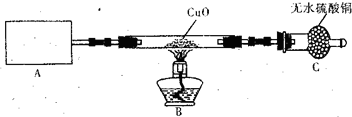
�ڼ���NH3�Ƿ��ռ����ķ�����_______________________________________________��
�۲�����ſ������ռ���һ��ƿNH3����������ͬ״������ͬ�����H2������10������������ƿ��NH3����Ȫʵ�飬ʵ����Ϻ���ƿ��ˮԼ��������ƿ�ݻ���__________(�����֮����)��
(1)��2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
�ڼ��ȵ������£�NaOH���Թ���ǿ�ҵĸ�ʴ����(��2NaOH+SiO2![]() Na2SiO3+H2O)
Na2SiO3+H2O)
�ƻ��������������ռ������Ĵ���(���ֹ������ˮ���������ռ�װ��)
���� ��ʯ��
(2)��CaO+NH3��H2O====Ca(OH)2+NH3��[��CaO+H2O====Ca(OH)2��NH3��H2O![]() NH3��+ H2O]
NH3��+ H2O]
���ò�����պȡ����ŨHCl����Բ����ƿ�ڣ����д������̣�֤���ռ�����(����ʪ��ĺ�ɫʯ����ֽ�����ռ�ƿ�ڣ�����ֽ������֤���ռ�����)
��![]()
ƽ��ʽ����10��2=20
 NH3ռ
NH3ռ![]() ��ˮ������
��ˮ������![]() ��
��