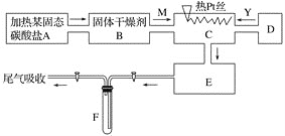
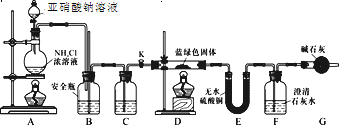
��Ŀ����
����Ŀ��A��B��C��D��E��F��G��H��I����ѧ��ѧ�г��������壬���Ǿ��ɶ�����Ԫ����ɣ������������ʣ�
��A��B��E��F��G��ʹʪ�����ɫʯ����ֽ��죬I��ʹʪ��ĺ�ɫʯ����ֽ������C��D��H����ʹʪ���ʯ����ֽ��ɫ��
��A��I����������ɫ������
��B��E����ʹƷ����Һ��ɫ��
�ܽ����ȵ�ͭ˿����װ��B��ƿ�У�ƿ�ڳ����ػ�ɫ���̣�
�ݽ���ȼ��þ������װ��F��ƿ�У�þ������ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱڸ��ź�ɫ������
��C��D�������ɺ���ɫ���壻
��G��D��ȼ�տ��Բ���E��H2O��
�ཫB��H��ƿ�л�Ϻ����������ü����ӣ�ƿ�ڱڳ�����״Һ�β�����A��
�ش��������⣺
��1��A�Ļ�ѧʽ��__________�����������Ļ�ѧʽ��______________��
��2�����з�����Ӧ�Ļ�ѧ����ʽ��____________________________________________��
��3�����з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��4��C�Ļ�ѧʽ��__________��D�Ļ�ѧʽ��__________��
��5�����з�����Ӧ�Ļ�ѧ����ʽ��____________________________________________��
��6��H�Ļ�ѧʽ��__________��
���𰸡�HClNH4ClCu��Cl2![]() CuCl22Mg��CO2
CuCl22Mg��CO2![]() 2MgO��CNOO22H2S��3O2
2MgO��CNOO22H2S��3O2![]() 2H2O��2SO2CH4
2H2O��2SO2CH4
��������
��A��B��E��F��G��ʹʪ�����ɫʯ����ֽ��죬Ϊ�������壬I��ʹʪ��ĺ�ɫʯ����ֽ������Ϊ�������壬IΪNH3��C��D��H����ʹʪ���ʯ����ֽ��ɫ������������ԣ�
��A��I�����������̣�ΪNH3��HCl��Ӧ�����Ȼ�泥���AΪHCl��
��B��E����ʹƷ����Һ��ɫ��ΪCl2��SO2��
�ܽ����ȵ�ͭ˿����װ��B��ƿ�У�ƿ�ڳ����ػ�ɫ���̣���BΪCl2��EΪSO2��
��Mg������F�о���ȼ�գ��к�ɫ�Ͱ�ɫ���ֲ����FΪCO2��
��C��D�������ɺ���ɫ���壬ΪNO��O2��
��G��D��ȼ�տ��Բ���E��H2O������EΪSO2����DΪO2��GΪH2S����CΪNO��
��BΪCl2����H��ƿ�л�Ϻ����������ü����ӣ�ƿ�ڳ�����״Һ�β�����A(HCl)����HΪCH4��
(1)������������֪��A�Ļ�ѧʽ��HCl���������ɵİ������Ȼ�泥���笠������������ӹ��ɣ���ѧʽΪNH4Cl���ʴ�Ϊ��HCl��NH4Cl��
(2)���з����ķ�Ӧ��ͭ��������Ӧ�����Ȼ�ͭ����Ӧ����ʽΪ��Cu+Cl2![]() CuCl2���ʴ�Ϊ��Cu+Cl2
CuCl2���ʴ�Ϊ��Cu+Cl2![]() CuCl2��
CuCl2��
(3)���з����ķ�Ӧ��Mg�ڶ�����̼��ȼ������̼������þ����Ӧ����ʽΪ��2Mg+CO2![]() 2MgO+C���ʴ�Ϊ��2Mg+CO2
2MgO+C���ʴ�Ϊ��2Mg+CO2![]() 2MgO+C��
2MgO+C��
(4)���������ӿ�֪��C�Ļ�ѧʽ��NO��D�Ļ�ѧʽ��O2���ʴ�Ϊ��NO��O2��
(5)���з����ķ�Ӧ������ȼ�����ɶ���������ˮ����Ӧ����ʽΪ��2H2S+3O2![]() 2SO2+2H2O���ʴ�Ϊ��2H2S+3O2
2SO2+2H2O���ʴ�Ϊ��2H2S+3O2![]() 2SO2+2H2O��
2SO2+2H2O��
(6)������������֪��H�Ļ�ѧʽ��CH4���ʴ�Ϊ��CH4��